Paraffin
(from Latin parum “little” + affinis “related”) is a waxy mixture of saturated hydrocarbons (alkanes) of predominantly normal composition from C18H38 (octadecane) to C35H72 (pentatriocontane).
Melting point – from 45°C to 65°C;
Density – 0.880-0.915 g/cm³ (15 °C);
When heated above 90°C, paraffin in air begins to steam intensely without boiling. Dense paraffin vapors heated to 120-150°C spontaneously ignite upon contact with air.
Obtained mainly from petroleum.
Depending on the ratio of the concentrations of heavy and light hydrocarbons, paraffin can be liquid, solid and finely crystalline (ceresin).
Discovered by Carl von Reichenbach.
Compound
Paraffins are a mixture of solid hydrocarbons of the methane series, predominantly of normal structure, with 18-35 carbon atoms per molecule and a melting point of 45-65 °C. Paraffins usually contain some isoparaffin hydrocarbons, as well as hydrocarbons with an aromatic or naphthenic core in the molecule.
GOST 23683-89 establishes 10 grades of paraffin (P1, P2, V1, V2, V3, V4, V5, T1, T2, C), differing in class and form of release:
- grades with index P are highly purified, intended for food production;
- brands with index B are highly purified with a narrower range of component weights, intended for non-food industries;
- grades with index T - purified technical paraffins;
- grade with index C is the so-called “match” paraffin, characterized by a high oil content and the lowest melting point.
The oil content in paraffin is standardized (0.8% for grade P2; 0.45% for all grades, excluding technical grades; over 1% for technical grades). The chemical composition of paraffins is standardized indirectly by melting point and microhardness (for grades of paraffins with the letter B, produced in the form of ingots).
How to make a candle: what you need and how to proceed
In addition to all the ingredients for the candles, you will need some additional materials and equipment. First, what you need for the candle itself: candle material, a wick with a holder. This is the minimum for a regular candle. Next is the fragrance and dye - for the colored aroma of the candle. This is enough to get started.
Making a handmade candle
What equipment do you need?
Melt the wax in a water bath. This should absolutely not be done over direct fire. For a water bath you will need two vessels of similar shape, but different sizes. For candle material, you can use a tin can of a suitable size, and water can be heated in any container. It is important that the container with candle material does not reach the bottom and remains “afloat”.
What do you need to make a candle?
When melting, the material cannot be overheated and cannot be underheated. It is necessary to bring it to the required temperature. So the temperature needs to be controlled. For this you will need a cooking thermometer. You also need a wooden or stainless steel stirring stick.
How and what to do
For beginner candle makers, it is better to prepare a candle mold in advance and secure the wick in it. Once again, please note that it must be strictly in the center. Place the finished form on an old saucer/plate/lid. This is in case something spills past or wax starts leaking out of an undetected hole.
First of all, secure the wick in the container
Pour water into a large container to half the volume and bring to a boil. We put the candle material in a jar or smaller container, put it in a water bath and wait until it starts to melt. As it begins to liquefy, add dyes, stir and monitor the temperature. As soon as it reaches the required level, remove the container from the water bath and cool slightly to the pouring temperature.
It is important to control the temperature
Just before you pour the wax into the mold, add flavorings and mix the composition again. Pour the mixture into the mold to the required level, but be sure to leave about 10% for topping up.
Hold the wick while pouring the wax, then fix it exactly in the middle
Leave the freshly cast candle to cool at room temperature for 8-12 hours. The exact time depends on the pouring temperature, wax, quantity/quality of dyes and flavors and other conditions. Do not try to speed up the process by placing it in the refrigerator, on the balcony or in another cool place. This leads to the appearance of defects. After it has cooled completely, you can try to pull out the candle if it is molded.
After cooling, depressions may appear near the wick
Some candles (usually stearin or with its additive) have larger or smaller depressions on the surface after cooling. This is a normal phenomenon and can be corrected by topping up. To do this, we left a certain amount of candle composition, rather than pouring it all out. After the candle has cooled, fill the crater with the molten residue and let it cool again. Larger depressions may require topping up twice or thrice.
Properties
the effect that occurs when mixing water with boiling paraffin
Paraffin is a white substance with a molecular weight of 300-450; in the molten state it has a low viscosity.
Paraffins are inert to most chemicals. They are oxidized by nitric acid, atmospheric oxygen (at 140 °C and above) and some other oxidizing agents to form various fatty acids, similar to fatty acids contained in fats of plant and animal origin. Synthetic fatty acids obtained by oxidation of paraffin are used instead of fats of plant and animal origin in the perfume industry, in the production of lubricants, detergents, and food products.
What to use for flavoring
To improve the aroma, aromatic substances are added to the candle base. In general, there are special fragrances for candles. It is easier to work with them if you are confident in the quality. Just add the recommended amount.
Set of scents for candles: Lemongrass, Jasmine, Flower bouquet. Recommended fragrance consumption is 6-12% of the total wax mass
For aromatization, special fragrances or aromatic oils with a high ignition temperature are used.
Many people prefer to create their own scents. In this case, you can use aromatic oils. These should be oils, not water or alcohol extracts. Another condition is that they must have a combustion temperature higher than 65°C. These are not all aromatic oils. Suitable oil extracts are geranium, peppermint, lemon mint, anise, amyris, myrrh, lemon myrtle, sandalwood, clove, rosewood, lemongrass, juniper, patchouli, ylang-ylang, lavandin (instead of lavender), patchouli, cinnamon, grape seed, bergamot .
Aroma oils are added to the already melted and colored composition when it has already begun to cool, before pouring. The quantity is counted in drops, the oils are added with vigorous stirring. It is impossible to indicate a specific number of drops. Some compositions have a more intense aroma and 3-5 drops per 100 grams of mass are enough, others are barely perceptible even with 20 drops.
Not all aromatic oils from all manufacturers have sufficient aroma, try finding another company.
Not all oil extracts in candles provide a sufficient level of aroma when burning. If you want a stronger scent, buy fragrances for candles or soap making. They generally smell stronger.
Some solid oils also have a pleasant smell. They can be used both as a base and as a flavoring. For example, coconut, shea, cocoa oils. When using them, standard candle bases account for about 20-30% in recipes.
Receipt
Paraffins can also be isolated from other products, for example, from ozokerite. Depending on the fractional composition, melting point and structure, paraffins are divided into liquid (tmelt ≤ 27 °C), solid (tmelt = 28-70 °C) and microcrystalline (tmelt > 60-80 °C) - ceresins. At the same melting point, ceresins differ from paraffins in having a higher molecular weight, density and viscosity. Ceresins react vigorously with fuming sulfuric acid, while paraffins react with it weakly. When distilling oil, ceresins are concentrated in the sediment, and paraffin is distilled with the distillate. Ceresins, which are concentrated in the residue after distillation of fuel oil, are a mixture of cycloalkanes and, in smaller quantities, solid arenes and alkanes. There are relatively few isoalkanes in ceresin.
Based on the degree of purification, paraffins are divided into the following types:
- slack and petrolatum, which contain up to 30% (wt.) oils;
- crude paraffins (ceresins) with an oil content of up to 6% (wt);
- purified and highly purified paraffins (ceresins).
Depending on the depth of purification, they are white (highly purified and refined brands) or slightly yellowish and from light yellow to light brown (unrefined paraffins). Purified paraffin has a density of 881-905 kg/m³. Ceresins are a mixture of hydrocarbons with the number of carbon atoms in the molecule from 36 to 55 (from C36 to C55). They are extracted from natural raw materials (natural ozokerite, as well as the remains of highly paraffinic oils obtained during its processing). Melting point 65-88 °C, molecular weight 500-700. Paraffins are widely used in electrical engineering, food (deep purification paraffins; tmelt = 50-54 °C; oil content 0.5-2.3% by weight), perfumery and other industries. Based on ceresin, various compositions are made in household chemicals, petroleum jelly; They are also used as thickeners in the production of greases, insulating materials in electrical and radio engineering, and wax mixtures.
Unrefined solid paraffins are produced using the following methods:
- deoiling of slack and petrolatum - by-products of the production (dewaxing) of oils using solvents (mixtures of ketone, benzene and toluene, dichloroethane), thereby obtaining crude paraffins (from slack) and ceresins (from petrolatum);
- separation and deoiling of paraffin from distillates of highly paraffinic oils using a mixture of ketone, benzene and toluene;
- precipitation of solid paraffins without the use of solvents (by cooling in crystallizers and filter pressing).
Crude paraffins are then refined (refined) using acid-base, adsorption (contact or percolation) or hydrogenation purification (to remove unstable substances that color and have an odor). Liquid paraffins are isolated from diesel fractions by dewaxing using selective solvents (a mixture of acetone, benzene and toluene), urea dewaxing (in the production of low solidification diesel fuel) and adsorption on molecular sieves (separation of C10-C18 liquid paraffins using porous synthetic zeolite).
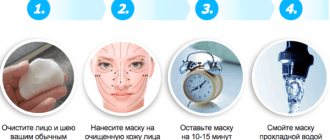
The mechanism of action of paraffin
When applying paraffin, the temperature in the underlying tissues increases by 1-3ºС. As a result, hyperemia occurs and increased blood flow at the site of paraffin application, metabolic processes improve, resulting in the following therapeutic effects:
- resorption of infiltrates,
- the inflammatory process is reduced,
- damaged tissues are restored,
- regenerative processes are activated,
- blood supply to problem areas improves,
- muscle spasms are relieved,
- the pain subsides,
- metabolic processes in the underlying organs and tissues improve.
As paraffin cools down, its volume decreases by up to 10%, while slightly compressing the skin and acting like a light massage.
The thermal effect occurs due to the slow cooling of the paraffin. The skin pores expand and sweat is released from them along with toxins and various waste products. Moreover, the moisture does not evaporate: it remains under the paraffin, leaving the skin moisturized. Harmful substances released with sweat are not absorbed back into the skin; their molecules are heavier than water, but remain on paraffin, which is then thrown away.
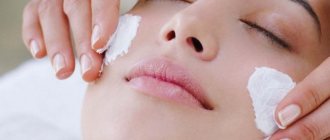
During the cosmetic procedure, microcirculation improves, making the skin moisturized and soft. As paraffin cools, it tightens the skin, making it immobile, and this helps smooth out wrinkles not only on the face, but also on the hands, which is now often observed even in young girls. When performing the procedure on the hands, paraffin helps strengthen the nail plates, protecting them from delamination and giving them strength and a healthy appearance.
Application
"waxing" is referenced here. A separate article is needed on this topic.
Not to be confused with initialing.
- candles for lighting and for making matches;
- lubricant for rubbing wooden parts (drawer guides, pencil cases, etc.);
- when mixed with gasoline - anti-corrosion coating (flammable!);
- in cosmetics for the production of petroleum jelly;
- paraffins are registered as food additives E905 x
; - used for paraffin therapy in medicine and cosmetology;
- lubricant for sliding cross-country skis, alpine skis and snowboards;
- in nuclear physics and technology: an effective neutron moderator and a proton “generator”;
- for lubricating bicycle chains;
- for greasing felt wads.
In radio engineering:
- For impregnation of electrical paper used in the manufacture of capacitors and winding transformers. Sometimes cardboard used to make circuit boards using the surface-mounting method is also impregnated.
- For filling frameless inductors, to protect them from vibrations and microphone effects. Often used, for example, in VHF radios. Sometimes the entire volume of the microassembly is filled.
- In other cases where high electrical strength, low AC losses, low price and the ability to be easily released from the filling by simple heating are required.
What to make a wick from
The most common type of wick used for candles is cotton. The easiest way to buy it is in a specialized store. If you do it yourself, you can twist it from cotton sewing threads, floss, or use cotton knitting thread of sufficient thickness. One thread is not enough for a candle wick. You can weave a braid from the threads, crochet it, or simply twist it into a rope. In principle, each type of wax requires its own type of wick and its diameter. Even for each composition, so they select the parameters empirically.
The thickness of the wick depends on the diameter of the candle
In any case, before pouring the candle, the cotton wick must be prepared:
- Melt some paraffin.
- Dip a piece of wick into it and leave for 5 minutes.
- Place on waxed paper or foil (it is important not to absorb fat) and level.
- Leave in the room for at least 6 hours or put in the refrigerator for an hour.
After such preparation, the wick will burn evenly. If you use it without preparation, there is a high probability that the flame will be “choked” as it will burn through too quickly.
In addition to thread wicks, there are wooden ones
There are also wooden wicks. They are made from wood veneer. They need to be dipped in oil, left there for a few minutes, placed on plastic or foil and kept for 6-8 hours. Wipe with a cloth before installation.
How to select the type and thickness of the wick
For long burning, the diameter of the wick is important. There is one important rule that beginners need to remember: the thicker the candle, the thicker the wick should be. But its specific diameter, and type, also depends on the composition of the base.
Table for selecting the diameter of the wick when casting a candle yourself
So, beeswax candles require a wick made of thicker threads. And the twist should be minimal. There may even be just folded threads, otherwise the fire will choke. For paraffin candles, make/choose wicks from thin, tightly twisted threads.
An incorrectly selected wick may cause the flame to go out.
How to choose a wick? By trial method. To make it easier, refer to the table above - it will help with your initial selection. Then, based on the combustion results, you can understand how to change the wick parameters.
- If the flame goes out due to a large puddle of wax/paraffin, the wick is too thin, try a slightly thicker one.
- If the flame smokes, the wick curls, and the candle leaks, you have used a wick that is too thick. Need something thinner.
Gradually, with experience, it will be easier to choose the right thickness and type of twist the first time.
How to install the wick
The uniformity and duration of burning of a candle largely depends on the wick. One of the important points is that it must be exactly centered and must be level. To install the wick in container candles, special holders are used. Wax is dripped into the center of the mold, and a holder with a thread attached is dipped into it. The second end of the thread is fixed exactly in the center on the upper edge of the form. This can be done using clothespins, twisting them around a pencil or holding them in hair clips. In principle, the method is not important. It is important that the wick is exactly in the center.
Using clothespins you can quickly and easily center the wick
In molded candles (which are removed from the mold after casting and cooling), you can attach the wick as described above. Another option is to make a hole in the bottom, pull the thread through and make a knot. Cover the hole with tape or drip some wax and wait until it hardens.
The threat of “paraffin poisoning” and its elimination
- main article Diesel fuel
- main article Dewaxing
In summer types of diesel fuel, a high paraffin content is often found. At temperatures below −5 °C, this causes crystallization of paraffin in the tank, as well as in all parts of the diesel engine from the tank to the injectors, which leads to diesel failure. To eliminate this, sometimes you have to clean the entire TA.
To prevent crystallization, kerosene, brake fluid, and special preparations commonly called antigel
. Also, paraffin from diesel fuel can be isolated during production by freezing and filtering. In Russia, the production of summer types of diesel fuel has not yet been prohibited, so in winter a lot of so-called “paraffin poisoning” occurs, which leads to failure and difficult repairs of diesel engines.
Contraindications for paraffin therapy
But not everyone can benefit from paraffin baths and applications. Significant harm to health is possible if there is a history of:
- acute inflammatory diseases or exacerbation of chronic diseases;
- coronary heart disease, exertional angina, severe atherosclerosis,
- chronic glamerulonephritis, liver cirrhosis, diabetes mellitus, high blood pressure;
- the presence of a large number of papillomas, moles and warts in the treated area;
- poor blood clotting;
- pregnancy and breastfeeding.
It is strictly forbidden to carry out such a procedure if there are any open wounds, ulcers, ulcers, eczema or weeping dermatitis on the skin.