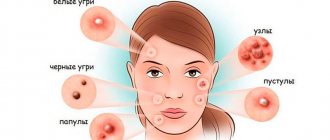
- Methods for diagnosing staphylococcal acne
- What is the treatment for staph infections and acne?
- Staphylococcus aureus and acne
- Treatment of Staphylococcus aureus and facial acne
- Who is at risk for staph and acne?
- Who is at risk of contracting staph?
- Is staph acne an infectious disease?
- How long are staph infections and acne contagious?
Staphylococcus (sometimes called "staph") is a group of bacteria that can cause a variety of conditions, including severe acne and pimples on various areas of the skin, including the face.
Staph infections can cause illness due to direct infection or due to the bacteria producing toxins. Pain, impetigo, food poisoning, cellulitis, and toxic shock syndrome are all examples of illnesses that can be caused by Staph.
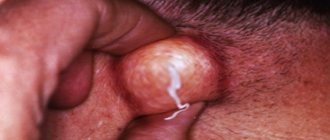
Symptoms and signs of a localized staph infection include clots of pus, such as in pimples, boils, boils, or abscesses on the skin. The area where the infection occurs is usually tender or painful and may be red and swollen.
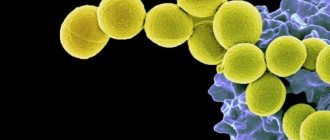
The name Staphylococcus comes from the Greek staphylos, meaning bunch of grapes, and kokko, meaning berry, and this is what staphylococcus bacteria look like under a microscope, similar to a bunch of grapes or small round berries.
Staph infections on the face or body may look like acne, but staph is a much more severe skin condition than regular acne. One way to tell the difference is that staph will not have a symmetrical border like a pimple or pustule. If you suspect you have If you begin to experience symptoms of staphylococcus, you should immediately consult a doctor to diagnose the relevant problem.
More than 30 different types of staph can infect people, but most infections are caused by Staphylococcus aureus Staphylococci are usually found in the nose and skin (and less frequently elsewhere) In most cases, the bacteria do not cause disease However, inflammation such as acne, a deep cut or other skin injury may allow bacteria to overcome the body's natural defense mechanisms, leading to further infection.
Staph infection is not the cause, but rather a complication of acne and pimples, especially in people who frequently break out. Acne can appear anywhere on the skin, most commonly on the face, neck, shoulders and upper back.
Methods for diagnosing staphylococcal acne
Blood tests are needed to diagnose staph when there is doubt or more serious infections occur.
In cases of minor skin infections and pimples, your healthcare provider can usually diagnose staph infections by their appearance without the need for laboratory testing. More serious staph infections, such as bloodstream infections, pneumonia, and endocarditis, require culturing samples of blood or infected body fluids or tissues.
The laboratory makes a diagnosis and performs special tests to determine which antibiotics are effective against such bacteria.
What is the treatment for staph infections and acne?
Poll: When did your acne appear? (Number of votes: 4295)
I've been suffering all my life
It's been a couple of years now
About a few months
Recently
To vote, click on the desired answer. results
Minor staph infections and skin pimples are usually treated with antibiotic ointment, such as Triple Antibiotic Blend, which is available over the counter. In some cases, oral antibiotics may be prescribed for skin infections. Additionally, if abscesses are present, they are surgically removed. More serious and life-threatening infections are treated intravenous antibiotics and supportive medical care in the hospital.
Doctors use several different types of antibiotics that have been used to treat staph infections. The choice of antibiotic depends on the type and severity of the infection, as well as the drug resistance patterns of the particular bacterial type. Some of the antibiotics that have been used to treat staph infections are cefazolin, cefuroxime, cephalexin, nafcillin ( nalpen), oxacillin (Bactocill), dicloxacillin, vancomycin, clindamycin (Cleocin), rifampin and telovacin (Vibativ) Combinations of antibiotics with other antibiotics can also be used. Some types of staphylococcus, such as MRSA, still remain resistant to many antibiotics.
Staphylococcus acne under a microscope photo
Staphylococcus aureus and acne
Staphylococcus aureus, which can accompany acne, is the most dangerous of all the common staph bacteria.
These bacteria are spread by direct contact with an infected person, using a contaminated object, or by inhaling infected droplets dispersed by a sneeze or cough.
Skin infections are common, and bacteria can spread through the blood and infect distant organs.
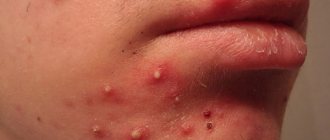
In addition to acne, a Staphylococcus aureus infection on the skin can cause blisters, abscesses, redness and swelling in the infected area.
Diagnosis is based on the appearance of skin or identification of bacteria in a sample of contaminated material.
Thorough hand washing can help prevent the spread of infection.
Antibiotics to treat the problem are chosen based on whether they can be effective against this type of Staphylococcus aureus, which causes an infection on the skin accompanied by persistent severe acne.
Staphylococcus aureus infection is more present in the nose, usually around 30%, and on the skin, around 20%. Percentages are higher for people who are hospitalized or work there, and for people prone to acne on the face and body. .
People who have the bacteria but do not have any symptoms caused by Staphylococcus aureus are called carriers. People who are most likely to be carriers include those whose skin has been repeatedly exposed to skin infections, such as:
- People with diabetes who must take insulin regularly;
- People who use drugs;
- People prescribed hemodialysis or chronic ambulatory peritoneal dialysis;
- People with skin infections, AIDS, or previous staph blood infections.
People can move bacteria from the nose to other parts of the body with their hands, sometimes triggering a more severe and active phase of the infection.
Staphylococcus aureus bacteria can spread from person to person through direct contact through contaminated objects (such as gym equipment, telephones, door handles, TV remote controls, or elevator buttons) or, less commonly, by inhaling contaminated droplets dispersed by sneezing or coughing.
Staphylococcus acne on the face photo
Literature
- Zipperer A., Konnerth MC, Laux C., Berscheid A., Janek D., Weidenmaier C. et al. (2016). Human commensals producing a novel antibiotic impair pathogen colonization. Nature. 535, 511–516;
- Dobson A., Cotter P. D., Ross R. P., Hill C. (2012). Bacteriocin production: a probiotic trait? Appl. Environ. Microbiol. 78, 1–6;
- Donia MS, Cimermancic P, Schulze CJ, Wieland Brown LC, Martin J, Mitreva M et al. (2014). A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell. 158, 1402–1414;
- Antimicrobial peptides are a possible alternative to traditional antibiotics;
- Lewis K. and Strandwitz P. (2016). Antibiotics right under our nose. Nature. 535, 501–502;
- Wikipedia: “Staphylococcus aureus”;
- Karpov I.A. and Kachanko E.F. (2005). Staphylococcal infection: clinical aspects and prospects for therapy. Medical news. 9, 53–56;
- Mandal A. (2012). What is Staphylococcus aureus? News-Medical.net;
- Naber C. K. (2009). Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin. Infect. Dis. 48 , S231–S237;
- Staphylococcus aureus (Staphylococcus aureus). GastroScan;
- Mobile genetic elements of prokaryotes: stratification of the “society” of vagabonds and homebodies;
- Wikipedia: “Non-ribosomal peptides”;
- Caboche S., Pupin M., Leclère V., Fontaine A., Jacques P., Kucherov G. (2008). NORINE: a database of nonribosomal peptides. Nucleic Acids Res. 36 , D326–D331;
- Mironovskiĭ ML, Ostash BE, Fedorenko VA (2010). Diversity of genes encoding nonribosomal peptide synthetases in the Streptomyces sioyaensis genome. Genetika. 46, 896–903.
Treatment of Staphylococcus aureus and facial acne
Antibiotics
Staphylococcus aureus infections are treated and treated with antibiotics. First, doctors try to determine whether the bacteria are resistant to antibiotics and, if so, which antibiotics.
The infection, which is acquired as a result of the development of pimples and acne, is treated with antibiotics that are effective against Staphylococcus aureus, such as: vancomycin, linezolid, tedizolid, Quinupristin plus dalfopristin, ceftaroline, telavancin, or daptomycin.
If testing results later indicate that the skin is sensitive to methicillin and the person is not allergic to penicillin, a methicillin-related drug such as nafcillin is used. Depending on how severe the infection is, a course of antibiotic treatment may be prescribed. which can last from several weeks.
Prevention of staphylococcal infections
Effective prevention of staph infections includes:
- Regular daily shower;
- Wearing comfortable, non-tight clothing made from natural fabrics without using synthetics;
- Don't share towels and razors with another person;
- Cleaning sports equipment before use;
- Avoid contact with infected people with pronounced purulent discharge and pimples;
- Avoid scratching the skin or squeezing pimples;
- Avoid wearing slippers in public showers and swimming pools;
- Wearing gloves when in contact with infected people.
Introduction
Two different types of microorganisms are present on the skin and appendages.
One of them consists of resident microorganisms or commensals, which, in most cases, are harmless and non-pathogenic, and sometimes beneficial to the host. The second group consists of transient microorganisms that can have harmful and pathogenic effects and colonize the skin after injury, which will lead to inflammation and the development of skin infection [1–3]. With the availability of bioinformatics technologies leading to the discovery of new phylogenetic approaches, many discoveries have been made in recent years. Studies have been conducted to study the diversity and topology of skin microbiota, to identify various commensal microorganisms present on the skin. This has made it possible to estimate the relative abundance of each population, and to understand their beneficial role or contribution to dermatological conditions such as acne[4–6].
Metagenomic analysis and 16S RNA ribosomal gene sequencing are the predominant bacteriological methods for analyzing the bacterial composition of microbial communities and for choosing the most effective study design, which is critical to obtaining meaningful analysis results [7].
Thus, the physiological characteristics of different skin sites have been associated with different levels of bacterial diversity, including Actinobacteria such as Propionibacteria, most recently renamed Cutibacteria (including Cutibacterium acnes, as well as Cutibacterium granulosum and Cutibacterium avidum), Proteobacteria, Bacteroides and Firmicutes; Staphylococcus epidermidis belongs to the latter group [5, 6, 8, 9].
Of these, C. acnes and S. epidermidis are the two main commensal skin bacteria [3, 10–12].
Until recently, research in the field of acne has mainly focused on the role of C. acnes, while the role of S. epidermidis has been discussed for several years but remains to be elucidated [10, 13].
The purpose of this article is to review the published data on the role of S. epidermidis in the physiopathology of acne and present future treatment prospects based on the available data and evidence.
Methods
A review of literature published between early 2000 and 2022 was performed. and available in PubMed using the following keywords: acne, acne vulgaris + microbiota, skin + microbiota, acne + microbiota + Propionibacteria acnes + Staphylococcus epidermidis, bacterial resistance, skin commensals + Propionibacterium acnes + Staphylococcus epidermidis.
results
Interaction between host skin and skin microbiota
The interaction of the host skin and microbiota in the skin immune system allows the differentiation of harmless commensal microorganisms, such as Corynebacterium sp., Staphylococcus sp. , excluding S. aureus, Cutibacterium sp., Malassezia furfur, and transient harmful pathogens such as S. aureus, Streptococcus pyogenes and Enterobacteriaceae. Even though recognition involves the human immune system, this mechanism is not fully understood. Toll-like receptors (TLRs) have been reported to be desensitized by long-term exposure to commensal microorganisms, or by decreased cell surface expression through activation of TLR kinase 3 pathway inhibitors [14, 15].
Moreover, specificity can be achieved by co-recognition of pathogen-associated molecules through their recognition receptors [6].
Thus, both such recognized commensals C. acnes and S. epidermidis interact with the host to help protect healthy skin from colonization by pathogens [9, 16].
Rice. 1. Healthy skin and skin with acne. A. Healthy skin. In healthy skin, S. epidermidis controls the proliferation of C. acnes. b Formation of microcomedone after excessive colonization of the skin by C. acnes, leading to dysbiosis. Excessive colonization with C. acnes during puberty leads to dysbiosis and acne. AMP, antimicrobial peptides; TLR, Toll-like receptors; PAR, protease-activated receptors.
However, the composition of the skin microbiota is constantly evolving and changing over time. For example, imbalance or dysbiosis of previously healthy skin, caused by external factors such as trauma, stress or pollution, or endogenous factors (hormonal changes, pH changes), can cause inflammatory skin diseases such as acne, atopic dermatitis, rosacea and psoriasis [ 13, 17–21].
In particular, during puberty, sebum production increases and its qualitative change occurs due to hormonal modifications, which can lead to the development of an unbalanced skin microbiota through increased colonization of the pilosebaceous complex and the skin surface with C. acnes [6].
This over-reproduction may be related to nutrient availability [22].
Role of C. acnes
In healthy skin, C. acnes plays a beneficial role in the microbiota of the pilosebaceous complex. It limits the growth of S. aureus, methicillin-resistant strains of S. aureus, and S. pyogenes while maintaining an acidic pH in the pilosebaceous complex by hydrolyzing sebum triglycerides and secreting propionic acid [6, 23, 24].
However, during puberty, excessive colonization of the pilosebaceous complex by C. acnes can lead to loss of diversity and dysbiosis, leading to the development of acne [6, 25–27].
A recent clinical study, using a single-locus sequence-based typing method, examined subgroups of C. acnes on the face and back of patients with severe acne and healthy controls [28].
Almost 75% of acne patients had identical C. acnes phylotypes on the face and back, whereas this was the case in only 45% of healthy controls. In the healthy group, phylotypes IA1 (39%) and II (43%) were the main phylotypes, whereas in the acne group, phylotype IA1 (84%), especially on the back (96%), was the main one. This may support the hypothesis that acne severity may be associated with loss of C. acnes phylotype diversity and selection of the major IA1 phylotype among acne patients [28–30].
Therefore, different inflammatory profiles depend on the phylotype (i.e., phylotype IA1, which is mainly observed on the face and back of acne patients), which activates innate immunity through the expression of protease-activated receptors (PARs), tumor necrosis factor-α and interferon-γ, and interleukins (IL-8) [28, 31–37].
In addition, C. acnes activates the release of lipases, matrix metalloproteinases and hyaluronidases, leading to hyperkeratinization of the pilosebaceous unit and, finally, comedones, papules and pustules [31–34, 38].
In Fig. Figure 1 shows the difference between a healthy microbiome and a microbiome with dysbiosis.
Role of S. epidermidis
S. epidermidis is the most commonly isolated commensal species from the epithelium [25, 39].
It colonizes predominantly the axillae, head, and nasal passages and is usually nonpathogenic to humans[11, 39].
S. epidermidis belongs to the group of coagulase-negative staphylococci, which differ from coagulase-positive staphylococci such as S. aureus in the absence of the coagulase enzyme. According to multilocus sequence typing, there is a large diversity of S. epidermidis, with more than 400 identified types (STs), compared to 155 for C. acnes [40, 41].
Most clinical isolates are CC2, from which ST2 is often isolated. It is possible that the successful spread of ST2 may be due to the fact that all ST2 isolates contain the IS256 sequence and ica genes, two factors that correlate with the invasiveness of S. epidermidis [42, 43].
However, there is still controversy as to whether icaA is a useful S. epidermidis biofilm marker [44].
In the past, S. epidermidis was considered a harmless commensal microorganism on human skin. It is currently considered an important opportunistic pathogen. It is the most common cause of hospital-acquired infections, as is its more virulent cousin, S. aureus. In particular, S. epidermidis represents the most common source of infection on stationary medical devices [45].
Treatment is complicated by specific antibiotic-resistant genes and the formation of biofilms, multicellular agglomerations that have intrinsic resistance and tolerance to antibiotics and host defense mechanisms [46].
In addition, studies have identified specific molecular determinants that allow S. epidermidis immune invasion and the ability to cause chronic diseases [47].
It is hypothesized that S. epidermidis has its own, original functions for a non-infectious lifestyle, highlighting the random nature of S. epidermidis infections, allowing the distinction between carriage, contamination and infection. A better understanding of the physiology of S. epidermidis is important for evaluating therapeutic strategies against S. epidermidis infections [39].
Some strains of S. epidermidis can modulate the host innate immune response, especially TLR 2, and thus enable the host to fight pathogens [6, 28].
Phenosoluble modulins produced by S. epidermidis have been shown to selectively inhibit skin pathogens such as S. aureus and group A Streptococcus, and together with host antimicrobial peptides (AMPs) enhance their clearance [48–50].
In addition, S. epidermidis activates keratinocyte AMP expression through a TLR2-dependent mechanism [51].
This confirms that this commensal interacts closely with the host innate immunity [48, 49].
This has also been shown through microdissection of epidermis, dermis, adipose tissue followed by 16S ribosomal RNA sequencing, demonstrating that S. epidermidis colonization regulates T cell trafficking and function through IL-1 [52, 53].
Another recent research paper reported that S. epidermidis may also protect against skin neoplasms by producing 6-N-hydroxyaminopurine, a molecule that inhibits DNA polymerase activity [54].
In addition, butyric acid secreted by S. epidermidis allows adipose tissue stem cells to differentiate into adipocytes and accumulate lipids in the cytoplasm, leading to an increase in the dermal layer [55].
Interaction between S. epidermidis and C. acnes in acne
S. epidermidis and C. acnes use glycerol as a common carbon source to produce various short-chain fatty acids (SCFAs) used as antimicrobial agents to compete with each other. These two microorganisms play a role in the pathogenesis and development of acne. [56, 57].
There is no evidence yet that S. epidermidis plays an active role in the pathogenesis of acne, but predominantly C. acnes is currently associated with acne [9].
Some isolates of S. epidermidis have been shown to have antimicrobial activity against C. acnes using antagonism assays [27, 56].
Among S. epidermidis strains with increased antimicrobial activity, differences in the diameter of the inhibitory zones were observed, indicating that the antimicrobial substances were of a different nature. Most S. epidermidis strains had small zones of inhibition (2–4 mm) against C. acnes, and some strains produced light-opaque zones of inhibition. One strain, FS1, produced very large inhibitory zones (>10 mm) but was not active against all C. acnes strains tested. Another strain, 14.1.R1, inhibited all strains of C. acnes but produced only small zones of inhibition (2–5 mm). There were no differences in the antimicrobial activity of S. epidermidis strains isolated from normal and acne skin, respectively. Similarly, the origin of C. acnes strains did not determine their susceptibility to the antimicrobial activity of S. epidermidis, since strains isolated from acne lesions and healthy skin did not differ significantly in their susceptibility patterns to S. epidermidis. On the contrary, the study showed higher antimicrobial activity among C. acnes phylogroup I-2, against S. epidermidis. The authors hypothesized the possible presence and secretion of a bacteriocin or bacteriocin-like substance specific to phylogroup I-2 of C. acnes [13].
The dependence of the severity of acne (mild, moderate or severe) on antimicrobial activity varied from 29 to 39%. Therefore, C. acnes type IA1 CC18 strains, mainly involved in acne, did not exhibit higher antimicrobial activity compared with healthy strains.
Various antagonism studies have shown that in vivo S. epidermidis controls C. acnes proliferation through the release of succinic acid, a fatty acid fermentation product that inhibits keratinocyte surface TLRs, tumor necrosis factor, and C. acnes-mediated IL-6 [27, 56, 58 ].
Since both C. acnes and S. epidermidis are present on the skin, suppression of inflammation caused by C. acnes may depend on the activation of the LTA miR-143 pathway on keratinocytes by staphylococci, which is necessary to reduce the inflammatory response. Studies have shown that the mechanism of LTA-miR-143-mediated suppression of TLR2 signaling is through miR-143 targeting the 3'UTR of TLR2. This reduces the amount of TLR2 protein and reduces inflammation caused by C. acnes [58–60].
Thus, it helps regulate skin homeostasis and suppress inflammation caused by C. acnes [58, 61].
Accordingly, an unbalanced balance between C. acnes and S. epidermidis in the pilosebacial units of acne patients in favor of the IA1 CC18 phylotype of C. acnes (75–80 cases) may prevent S. epidermidis from fully playing its role as a regulator of natural skin homeostasis and limiting the growth of C. acnes. Figure 2 visually shows the interaction between C. acnes and S. epidermidis.
Figure 2. Bacterial interactions on the skin. AMP, antimicrobial peptides.
S. epidermidis and its implications for current and future acne treatments
Current topical acne treatments include various retinoids, such as adapalene and tretinoin, which reduce inflammation by altering the innate immunity activated by C. acnes [62–65].
In addition to benzoyl peroxide, the use of antibacterial agents such as erythromycin and clindamycin in monotherapy resulted in the development of antibiotic-resistant strains of not only C. acnes but also S. epidermidis within 4–6 weeks [30, 57, 66].
Systemic antibiotics have been reported to induce only minor resistance but may lead to adverse effects in the gut microbiota [30, 33, 67].
Oral isotretinoin normalizes the innate immune system response to C. acnes by inhibiting its proliferation [68].
Thus, eliminating C. acnes alone may promote the proliferation of S. aureus, causing skin inflammation and proliferation of S. epidermidis, leading to other unbalanced skin homeostasis and the risk of nosocomial infections.
AMPs are effector molecules of the skin's innate immune system. They are amphipathic (both lipophilic and lipophobic) and destroy the lipid membrane of the bacterium, leading to lysis and cell death, interacting preferentially with negatively charged bacterial membranes rather than with neutrally charged membranes of mammalian cells. The importance of the contribution of AMPs to human immunity is undeniable, since changes in AMP expression are associated with various pathological processes. However, data on the role of AMPs in acne vulgaris are limited. A recently published study reports that the AMPs hBD-1 and cathelicidins play an important role in the pathogenesis of acne [69].
AMPs have shown activity against a wide range of Gram-positive and Gram-negative bacteria, as well as some fungi, parasites and enveloped viruses [70].
They are produced as a response of keratinocytes and sebocytes. However, they also contribute to the development of additional inflammatory reactions. AMP production includes β-defensins, RNase 7, psoriasin S100 protein, and cathelicidins and is mediated by the MyD88 pathway and IL-1 signaling. Additionally, it has been demonstrated that more cells express TLR-2 as acne severity increases[33].
This may be one explanation why agents targeting TLR-2, such as topical retinoids, are more effective in patients with more severe acne [71].
Cytokines are also produced as a result of the interaction between C. acnes and TLR-2, defensins and matrix metalloproteinase through activation of PAR-2R [72].
Another treatment approach may be regular oral or topical supplementation of the skin microbiota with beneficial microorganisms (probiotics) for acne patients to balance the skin microbiota [73, 74].
In 2010, Arck et al. [75] suggested that there is not only a gut-brain or skin-brain axis, but also a gut-skin axis. The authors showed that oral supplementation of a Lactobacillus strain in mice attenuated stress-induced neurogenic skin inflammation and inhibited hair loss. Their concept suggested that modulation of the microbiota through the use of probiotics reduces neurogenic skin inflammation. These observations provide hope that the right type of bacteria may have beneficial effects on skin homeostasis, inflammation, and the response of peripheral tissues to perceived stress. Other human studies have confirmed this hypothesis [76–78].
Thus, probiotics may be effective in acne and other inflammatory skin diseases such as atopic dermatitis and possibly psoriasis [79–83].
From this point of view, studies show that S. epidermidis can control dysbiosis caused by C. acnes and reduce the severity of acne [27, 56].
However, it remains unclear which SCFA in the glycerol fermentation products of S. epidermidis primarily contributes to anti-C. acnes effect. It is also not determined whether SCFAs act together with other antimicrobial molecules in fermentation products to provide anti-C. acnes effect. Anti-S. acnes effect of the fermented medium persisted after boiling the said medium, suggesting that antimicrobial proteins/peptides may not be a major contributor to anti-C. acnes effect of fermented media [56].
Prodrugs such as SCFA pivaloyl methylbutyrate (AN-9) have been developed to achieve pharmacological concentrations of such SCFAs in vivo [84].
Moreover, live S. epidermidis can potentially be used as an active component in probiotics for acne[27, 56].
Discussion and conclusion
Acne is a chronic and multifactorial inflammatory disease of the skin and pilosebaceous unit. Dysbiosis in acne patients is associated with decreased numbers of S. epidermidis and overcolonization of certain C. acnes phylotypes in the pilosebaceous unit, resulting in varying levels of innate immune activation and varying degrees of acne severity. Recent studies appear to support a beneficial role of S. epidermidis in acne physiopathology by limiting C. acnes skin colonization and inflammation [9, 33].
However, an imbalance in favor of S. epidermidis may also lead to other health consequences, such as hospital-acquired infections. Therefore, balanced skin homeostasis should be the ultimate goal of any acne treatment.
From this perspective, other treatment options can be considered, such as S. epidermidis-derived probiotics to restore a natural, balanced microbiota, and regulation of host AMP mediators without increasing the local epidermal S. epidermidis population.
Who is at risk for staph and acne?
- Nurses, doctors, patients with diabetes, and hemodialysis patients are particularly at risk;
- Newborns;
- Women who are breastfeeding;
- Obese people;
- People living in crowded communities or hot climates;
- Gymnasts sharing exercise equipment, people going to the gym;
- Persons with skin injuries, surgical wounds, piercings and fresh tattoos;
- People with atopic dermatitis or seborrhea, a compromised immune system, cancer, diabetes, blood or lung diseases, including those treated with steroids or chemotherapy
- People prone to the appearance and rapid development of acne, which can develop into a more severe form of acne under the influence of staphylococcus, are especially at risk.
How to quickly remove and relieve acne inflammation overnight on the face and body?
Secret weapon
The product of the lug operon isolated by Zipperer turned out to be a non-ribosomal cyclic peptide consisting of five amino acids (two D-valines, L-valine, D-leucine and L-tryptophan) and a thiazolidine heterocycle (Fig. 4). The antibiotic was named lugdunin.
Figure 4. Gene cluster, biosynthetic pathway, and chemical structure of lugdunin. a — Genes of “subunits” (not modules!) of non-ribosomal peptide synthetase of S. lugdunensis: lugA, B, C and D. b — Functional domains of operon products: A - adenylating, P - peptidyl, C - condensing, E - epimerizing, R - reductase. Their specific combinations make up modules—the individual catalytic units of an enzyme. The biosynthesis of lugdunin apparently begins in the initiator module LugD and continues sequentially with the help of LugA-C. c — Structural formula of lugdunin.
[1]
By chemical synthesis, it was possible to obtain a product with chemical properties and antibacterial effect identical to natural lugdunin. Scientists have suggested that this antibiotic inhibits the synthesis of bacterial biopolymers - proteins, DNA and peptidoglycans [5].
Non-ribosomal peptides
This class of peptides is synthesized in the cells of lower fungi and bacteria without the participation of ribosomes. Non-ribosomal peptides (NRPs) are also found in higher organisms that have commensal bacteria [12].
NRPs are divided into several functional groups [13]:
- antibiotics (vancomycin);
- antibiotic precursors (ACV tripeptide - a precursor to penicillin and cephalosporin);
- immunosuppressants (cyclosporine);
- antitumor peptides (bleomycin);
- siderophores (pyoverdine);
- toxins (HC-toxin);
- surfactants (surfactin).
Structure
Nonribosomal peptides range from 2 to 50 amino acids in length and often have a cyclic or branched structure. They contain both “regular” proteinogenic and non-proteinogenic amino acids - D-forms or residues modified by the addition of N-methyl and N-formyl groups, glycosylation, hydroxylation, acylation or halogenation. Cyclization occurs through the formation of oxazolines and thiazolines in the peptide backbone [12].
Synthesis
NRPs are synthesized by non-ribosomal peptide synthetases (NRPS), which do not follow “foreign” instructions in their work, that is, they do without mRNA. NRPS are giant multimodular enzymes, each of which can synthesize only one type of peptide. A separate enzyme module is responsible for the inclusion of one amino acid in the peptide chain; therefore, the number of modules corresponds to the length of the peptide [14].
Each module consists of at least three domains:
- condensing (accepting the peptide chain from the previous module);
- adenylating (selecting the desired amino acid);
- peptidyl (forming a peptide bond).
Modules often include other domains, including the epimerizing domain, which converts L-amino acids into D-forms [14].
By analogy with the triplet ribosomal code for protein synthesis, there is also a non-ribosomal NRPS code, determined by 10 amino acid residues in the substrate-binding pocket of the adenylation domain. The combination of these residues determines which amino acid will be integrated into the peptide by a specific NRPS module. Knowing this code, it is possible to predict the substrate specificity of adenylation domains and even arbitrarily change it by replacing amino acids in the domain [14].
In experiments by German scientists, lugdunin acted not only on methicillin-resistant staphylococci, but also on glycoprotein-resistant, and even on other gram-positive bacteria such as listeria and vancomycin-resistant enterococcus (Table 1).
The minimum inhibitory concentration (MIC) of the new bacteriocin is 1.5–12 μg × ml−1, which indicates the high activity of the substance. However, such concentrations had no effect on human serum, did not cause lysis of neutrophils or red blood cells, and did not inhibit the metabolic activity of monocytes. Bacterial cells exposed to lugdunin at concentrations even below the MIC stopped synthesizing DNA, RNA, proteins and cell wall components. In this respect, lugdunin is similar to daptomycin, which has the same effect, but whose mode of action has not yet been studied. No resistance of S. aureus cells to lugdunin was observed even after they had been grown for a month at low concentrations. Table 1. Spectrum of activity of lugdunin
| Types and strains | Resistance | MIC of lugdunin (μg × ml−1) |
| Staphylococcus aureus USA300 (LAC) | MRSA | 1,5 |
| + 50% human serum | 1,5 | |
| Staphylococcus aureus USA300 (NRS384) | MRSA | 1,5 |
| Staphylococcus aureus Mu50 | GISA | 3 |
| Staphylococcus aureus SA113 | 3 | |
| Staphylococcus aureus RN4220 | 3 | |
| Enterococcus faecium BK463 | VRE | 3 |
| Enterococcus faecalis VRE366 | VRE | 12 |
| Listeria monocytogenes ATCC19118 | 6 | |
| Streptococcus pneumoniae ATCC49619 | 1,5 | |
| Bacillus subtilis 168 (trpC2) | 4 | |
| Pseudomonas aeruginosa PAO1 | >50 | |
| Escherichia coli DH5α | >50 | |
| Legend: MRSA - methicillin-resistant S. aureus; GISA—glycoprotein-resistant S. aureus; VRE—vancomycin-resistant Enterococcus. Table from [1]. | ||
Who is at risk of contracting staph?
Anyone can develop a staph infection, although some groups of people are at greater risk, including newborns, women who are breastfeeding, and people with chronic medical conditions such as diabetes, cancer, vascular disease, and lung disease. Injecting drug users who have skin injuries. or disorders, intravenous catheters, surgical incisions, and people with a weakened immune system caused either by disease or as a result of immunoconverting drugs all have an increased risk of developing staph infections.
Certain groups of people are at greater risk of staph infection, such as newborns, diabetics, injection drug users, and surgical incision patients.
About the disease
Staphylococcal infection combines a number of diseases that can manifest as purulent lesions of the child’s skin, pathology of the digestive tract, involvement of ENT organs or even bones in the process.
It is important to consider that the severity of the clinical picture and the danger of the pathology for the patient directly depends on the type of staphylococcus that caused the inflammation. The disease develops sporadically (unsystematically). The risk of infection largely depends on the baby's immune defense. Traditionally, there has been an increase in the number of episodes of staphylococcal infection in newborns. This is due to insufficient secretion of the natural factor IgA, which is responsible for protecting the mucous membranes throughout the human body.
The most common types of staphylococcal damage to the body are:
- digestive disorder caused by staphylococcus;
- septicemia (spread of bacteria in the blood);
- staphylococcal tonsillitis (angina);
- osteomyelitis.
The specialists of the SM-Doctor clinic have many years of experience in successfully treating children of any age who are faced with diseases caused by staphylococcus.
Is staph acne an infectious disease?
Staph infections are contagious until the infection resolves. Direct contact with an infected sore or wound or with personal care items such as razors, bandages, etc. are common ways of transmitting the infection. Casual contact such as kissing or hugging. pose little risk of transmission unless there is direct contact with the infected area.
Some types of staph infections (see below) include staph, which causes food poisoning or toxic shock syndrome. These specific staph bacteria cause illness by producing a toxin. The toxin is not contagious, but food poisoning can affect groups of people who eat the same contaminated food. .