Psoriasis is a chronic, non-infectious skin disease that affects the skin, nails and joints. There are several theories of the origin of the disease - viral, psychoneurogenic, autoimmune, hereditary, etc., but none of them is generally accepted. Scientists have come to the conclusion that psoriasis has a multifactorial nature.
According to some data, more than 3% of the world's population suffer from psoriasis on the face and body, and the disease can appear for the first time at almost any age.
Until now, the disease was considered incurable, but techniques have emerged that will not only bring noticeable relief to the patient, but also ensure stable remission of psoriasis. Treatment is based on the use of innovative technologies.
Psoriasis is successfully treated in our clinic. Treatment of this and other dermatological diseases (vitiligo, atopic dermatitis, eczema) is carried out using the latest high-tech equipment. We know how to significantly reduce the manifestations of psoriasis and achieve stable remission even in the most difficult cases.
In addition, treatment with the Excilite µ excimer system allows the patient to stop taking steroid (hormonal) drugs.
1 Excilite µ system
2 Excilite µ system
3 Excilite µ system
Causes of psoriasis on the face
Psoriasis on the face is rarely isolated; usually, similar rashes are present on the skin of the upper and/or lower extremities and torso.
It has been statistically proven that facial skin lesions in people diagnosed with psoriasis occur in 42% of patients. Such indicators indicate a fairly widespread change. Before the problem began to be closely studied, it was believed that psoriasis on the face was characteristic of the Caucasian race, as it manifests itself several times more often. This assumption is explained by weak exposure to sunlight and cold, in contrast to the population of African countries, the South American continent, Eskimos and Japanese.
How and from what causes psoriasis on the face has not been precisely established; we can talk about several combined causes or one associated with a malfunction of the immune system, a change in a section of a chromosome, etc. Reasons for the development of psoriatic rashes:
- Hereditary and genetic predisposition. It has been established that the likelihood of developing a skin disease is higher in people whose immediate relatives suffer from psoriasis. Several genes have also been identified that are responsible for the formation of a predisposition to the development of psoriasis (PSORS 1-13). Changes in the functioning of these genes lead to the development of an inflammatory response, disruption of the cell cycle, defects in the barrier function of the skin, etc.
- Immune system dysfunction. In psoriasis, there is also a malfunction in the functioning of a number of immune cells (T-lymphocytes, antigen-presenting cells and others) and signaling molecules (in particular, interleukins and interferons) involved in the development of the inflammatory process.
- Prolonged exposure to a stress factor or severe nervous shock.
- Severe and moderate course of infectious diseases.
- Skin injuries, especially professional injuries affecting the same area of the body (Koebner phenomenon).
- Taking a number of medications.
- Smoking and drug abuse, unhealthy diet.
There are different classifications of psoriasis; a number of researchers divide the disease into 2 types depending on the age when the rash first appeared:
- Patients under 40 years of age, peak detection period is 16-22 years. Characterized by rapid spread of the rash, generalized nature, difficult to control course and frequent relapses.
- Patients are over 40 years old, peak – 57-60 years. There are mild symptoms, a sluggish process, and is easily amenable to pathogenetic therapy.
Psoriasis. Treatment
Such a varied clinical picture of the disease makes diagnosis difficult - it is necessary to differentiate psoriasis from a significant number of diseases (for example, lichen planus, syphilis, seborrheic dermatitis, atopic dermatitis , rheumatoid arthritis , etc.).
Remember that the slightest peeling or redness of the skin cannot be ignored - these may be symptoms of psoriasis and other dermatological diseases that will progress without treatment. Experienced dermatologists at our clinic will conduct the necessary research and make the correct diagnosis.
Treatment of psoriasis is aimed at eliminating the inflammatory process and includes a whole range of therapeutic measures. Today there are many methods and drugs for the treatment of poriasis. When prescribing therapy, it is important to take into account the prevalence of skin lesions, the form and stage of the disease, the characteristics of the process, the presence of concomitant diseases, etc.
The comprehensive treatment program for psoriasis includes general and local therapy, the prescription of antihistamines, physiotherapeutic methods, etc.
Local drug therapy (using products containing sulfur, naphthalan, tar and other components) is aimed at reducing inflammation and peeling of the skin.
In the progressive stage, glucocorticosteroids are prescribed, the use of which has a number of restrictions and should be prescribed exclusively by a specialist. Thus, the use of strong glucocorticosteroids is associated with a high risk of withdrawal syndrome, which is expressed in a sharp exacerbation of the process. Self-medication with such drugs can lead to atrophy, swelling of the skin, etc.
1 Excilite µ system
2 Excilite µ system
3 Excilite µ system
We know how to treat psoriasis! The MedicCity clinic uses the latest Excilite excimer laser system. Unique equipment makes it possible to achieve stable remission of the disease and a significant reduction in the manifestations of psoriasis.
It is very important to start treating psoriasis as early as possible! You can check prices for a course of procedures with the clinic administrator or by phone!
1 Excilite µ system
2 Excilite µ system
3 Excilite µ system
Treatment of psoriasis using the Excilite system is based on exposure of the affected area to monochromatic radiation with a wavelength of 308 nm. There is a targeted phototherapeutic effect on pathological cells. The effectiveness of treatment is 90%! Positive results are noticeable after just a few procedures. Rapid healing of inflammatory foci and removal of plaques occurs. Moreover, the procedures are painless, comfortable, and have no side effects.
1 Excilite µ system
2 Excilite µ system
3 Excilite µ system
In the treatment of psoriasis, it is also important to follow a special diet, special skin care (the use of moisturizing, skin-softening creams, etc.), spa treatment, the use of vitamin therapy, etc.
In addition, it is necessary to correct concomitant diseases of the gastrointestinal tract, nervous system, hormonal sphere, etc.
The material was prepared with the participation of a specialist:
Photo of psoriasis on the face, what psoriasis looks like on the face
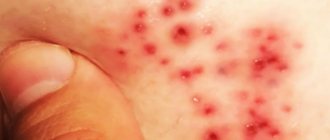
Photo 1. Psoriasis on the face.
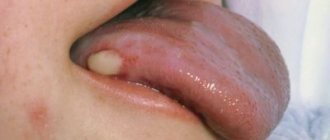
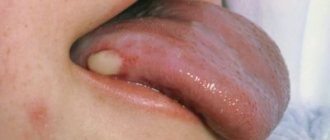
Photo 2. Psoriasis of the scalp.
Photo 3. Seborrheic psoriasis of the face.
Photo 4. Psoriasis on the skin of the face.
Psoriasis on the face in the initial stage may resemble simple dermatitis; for comparison, just look at the photo on a medical website. In order to get acquainted with what classic psoriasis looks like on the face, it is recommended to familiarize yourself with the photo.
Differences from other diseases
In infants, psoriasis is similar to regular diaper rash. It is for this reason that parents often do not consult a doctor on time. This dangerous disease is confused with diaper rash and a common rash, which is a common companion in infancy. However, psoriasis has a characteristic feature that distinguishes it from other harmless rashes on children's skin. Let's call these differences:
- the horny ball of papules (nodules on the skin) exfoliates, peeling occurs;
- the skin becomes crusty;
- isolated small nodules-papules unite into formations of significant size;
- the size of the nodules is continuously increasing. Such associations occupy large areas of the skin over time.
Symptoms of psoriasis on the face
The intensity of the manifestation of skin symptoms on the face differs from how and at what age psoriasis begins. At a young age, the rashes are typical, they are difficult to confuse with other types of dermatitis, unlike in older patients.
Psoriatic rashes are flat papules or plaques that rise above the skin, have clear outlines and a rounded shape. The rashes are usually bright pink in color and tend to merge. On the surface of the elements, multiple white scales are dotted, which easily fall off when rubbed and scraped.
The rash may spontaneously regress, leaving slight pigmentation on the skin.
The rash can be located on any part of the face, the most typical areas being the border with the scalp, the skin of the eyelids, nasolabial folds, and the area of the beard and mustache.
The formation of plaques occurs in several stages; first, new pink elements appear, which are partially covered with scales. The plaques unite and white-silver scales are observed over the entire surface, which is characteristic of stopping the growth of the rash. Regression of rashes is accompanied by a decrease in local redness, infiltration, and peeling.
In dermatological practice, there are specific clinically significant symptoms characteristic only of psoriasis. Psoriatic triad:
- Symptom of a stearin stain: when scraping with a glass slide over the rash, the peeling intensifies.
- The appearance of the terminal film: when the scales are removed, the skin is bright red, smooth, and shiny.
- Auspitz's sign (blood dew): scraping the terminal film leads to pinpoint bleeding due to closely spaced capillaries.
Types of psoriasis
- Simple. Diagnosed in 85% of cases. There is redness in areas of the skin with white scales. This type of psoriasis is often found on the scalp. The skin under the scales is easily injured; wounds can bleed and cause pain.
- Back. Appears in folded areas: under the breasts in women, in skin folds, armpits, inner thighs, external genitalia.
- Pustular. This type of psoriasis causes clear blisters to form. There is red, inflamed skin around the pustule.
- Rupeeodny. In addition to the presence of plaques, this species is characterized by acute inflammation of the epidermis. The skin under the plaques is pink and wet.
- Teardrop-shaped. It is distinguished by a large number of small bubbles of red or purple color. They are shaped like dots or drops. This type of psoriasis appears on the arms, head, neck, shoulders, back, and thighs.
Is psoriasis transmitted?
This is a common question that concerns patients and their families. The disease is not contagious, since its cause is not associated with pathogenic microorganisms. It is assumed that psoriasis can only be transmitted genetically, that is, when there is a genetic predisposition. Psoriasis on the nails is often a concern for healthy people. After all, the patient touches any surface. However, there is no need to worry.
Treatment of psoriasis on the face
Before you begin to treat psoriasis on the face, you will need to collect a full history of the patient’s life and illness, perform laboratory and instrumental diagnostic procedures, it is important to establish the degree of activity, type and presence of complications for individual selection of therapy.
Treatment of psoriasis on the face with oral and parenteral medications is prescribed in the case of a generalized, long-term or advanced form. For such treatment, cytostatics and immunosuppressants are mainly used,
Topical remedies for psoriasis on the face are divided into hormonal and non-hormonal. It is important to note that hormonal liniment drugs have a quick, but not always long-lasting effect. In addition, long-term application of active and highly active corticosteroids can cause skin atrophy and decreased visual acuity in psoriasis on the eyelids.
Corticosteroid ointments and creams are prescribed in a short course in combination with a vitamin D3 analogue or salicylic acid, which enhances the therapeutic effect.
Non-hormonal ointments and creams for the treatment of psoriasis on the face:
- Salicylic ointment in low concentration (2-5%) relieves inflammation and peeling.
- Tar-based preparations help reduce inflammation and itching and affect the rate of cell division.
- Kartalin has an antiphlogistic effect, regulates the division and maturation of epidermal cells.
- Drugs containing grease in their composition also have similar effects.
Treatment
If psoriasis is detected in children, treatment must be started as early as possible. Its success and comfort for the child depend on this. If you contact a pediatric dermatologist as quickly as possible, this increases the chances of persistence and duration of remission.
If the presence of the disease has been established, hospitalization and inpatient treatment are carried out. However, such measures are only necessary for the first application. Then hospitalization will no longer be required. To help the child get rid of itching, antihistamines plus calcium-containing drugs are prescribed. If the disease has spread sufficiently throughout the body and the skin lesions are extensive, hormonal medications may be prescribed.
You can treat the affected areas with baby creams and ointments prescribed by your doctor. You may also be prescribed to take baths and perform water procedures as often as possible. When psoriasis is detected in children, the causes and treatment are interrelated. Doctors are now inclined to believe that the cause of the disease may be autoimmune. Therefore, drugs are prescribed in connection with this assumption of the appropriate type. These are immunosuppressants - drugs that can suppress the activity of immune processes. A diet is prescribed that excludes the consumption of animal fats. With its help, you can significantly alleviate the condition of a small patient in just a week.
Losterine in the treatment of psoriasis on the face
For patients suffering from psoriasis on the face, the manufacturer of the Losterin line has created special care products based on deresined naphthalan:
- Cream.
- Shampoo.
- Shower gel.
- Cream soap
The peculiarity of the Losterin line is the possibility of use during periods of exacerbation and remission as complex therapy. The drugs are characterized by a balanced composition. The ingredients are selected so that the action of one component is potentiated and complemented by another. Gently soothe the skin, relieve signs of inflammation, moisturize, and promote rapid healing.
Causes of the disease
Guttate psoriasis is not a contagious disease. Since the nature of the disease is non-infectious, it is impossible to become infected through contact with a patient with drip psoriasis. Scaly rashes on the skin appear as a result of autoimmune processes in the body. The immune system aggressively attacks its own cells, mistaking them for foreign bodies. The exact reasons for the launch of autoimmune processes have not yet been established.
There may be several exogenous risk factors for the appearance of a teardrop rash:
- Unhealthy Lifestyle;
- action of aggressive chemicals;
- poor ecology of the region of residence;
- psychological overload.
Possible endogenous causes of the disease include:
- bacterial infections;
- viral diseases;
- genetic predisposition;
There are many reasons for the appearance of the disease, so the number of people with guttate psoriasis is growing every year all over the world, and in particular in Russia.
Prevention of psoriasis on the face
Preventive measures are aimed at preventing the development of the disease or the transition of psoriasis from remission to the acute stage. They imply adherence to a daily routine and rest, proper and balanced nutrition, giving up bad habits, and eliminating stress factors.
Basic criteria for caring for delicate facial skin:
- wash only with special moisturizers;
- water at a comfortable temperature (30-36°C);
- soak the remaining moisture with a soft towel;
- the use of creams that protect against cold and ultraviolet rays;
- wearing clothes (outerwear with a hood, scarf) and accessories according to the season (use an umbrella in rain and wind);
- Men are recommended to use an electric razor.
Compliance with preventive measures and timely therapy prescribed by a specialist will reduce the risk of spread and relapse of the disease.
What is selective phototherapy
This physiotherapeutic method uses mid-wave ultraviolet rays. Their effect is extremely beneficial, and is manifested in the complete disappearance of psoriasis, and the treatment has practically no contraindications.
For use, both narrow-band and broadband units are used that generate ultraviolet radiation, making it possible to act strictly on rashes without affecting healthy areas. Specialist dermatologists at the Moscow Aloderm clinic, using the latest generation technologies, achieve lasting results, eliminating the manifestations of psoriasis and will allow you to abandon the constant use of hormonal ointments.
Biological drugs created using genetic engineering methods are monoclonal antibodies used for therapeutic purposes. In domestic medical practice, the following biological drugs are approved for medical use for the treatment of psoriasis: infliximab, adalimumab, ustekinumab, etanercept.
1. Infliximab
Infliximab is a selective TNF-a antagonist, which is a chimeric monoclonal IgG antibody that consists of 75% human and 25% mouse protein. Infliximab is indicated for the treatment of adult patients with psoriasis with severe and moderate forms of the disease in the absence of clinical effect from the use of other systemic therapies (including cyclosporine, acitretin, methotrexate and PUVA therapy) or in cases of intolerance or contraindications to their use, as well as for the treatment of active progressive psoriatic arthritis.
Doses and regimens
The drug is administered intravenously by drip for at least 2 hours at a rate of no more than 2 ml/min. under the supervision of medical personnel. For the treatment of psoriasis and psoriatic arthritis, the initial dose of infliximab is 5 mg per kg of patient weight. After the first administration, the drug is administered in the same dose after 2, then 6 weeks. and then every 8 weeks. If there is no effect within 14 weeks. (after four intravenous infusions) it is not recommended to continue treatment. During the intravenous infusion and for at least 1-2 hours after its completion, the patient must be under the supervision of a physician. During intravenous infusion of the drug, it is necessary to measure blood pressure, pulse, and respiratory rate and body temperature every 30 minutes.
Adverse reactions/safety
In clinical studies, adverse reactions were observed in approximately 60% of patients receiving infliximab and 40% of patients receiving placebo.
- Infusion reactions occur during infusions or within 1-2 hours after it. These include swelling of the pharynx/larynx, bronchospasm, chills, headache, hot flashes, nausea, shortness of breath. In clinical trials, the incidence of infusion reactions when using infliximab was about 20%, in the comparison group (placebo) - about 10%. Approximately 3% of patients were forced to discontinue treatment due to the development of infusion reactions, which were reversible in all patients (with or without drug therapy).
- Delayed hypersensitivity reactions in the form of arthralgia, myalgia, fever and rash develop in 1% of patients with psoriasis at the beginning of treatment.
- In clinical trials, when infliximab treatment was repeated 2-4 years after the previous course of therapy, patients experienced adverse reactions (myalgia, arthralgia accompanied by fever and/or rash, itching, swelling of the face, lips or hands, dysphagia, urticaria, pain sore throat and/or headache), which developed 3-12 days after re-infusion.
- Infectious complications are the most common serious side effects. They were associated with approximately 50% of all recorded deaths. There have been cases of tuberculosis, including miliary tuberculosis with extrapulmonary localization, in some cases with a fatal outcome.
- Malignant neoplasms and lymphoproliferative diseases. There have been cases of emergence or recurrence of malignant neoplasms. The incidence of lymphoma in patients treated with infliximab was higher than the expected incidence of this disease in the general population. The incidence of other forms of malignant neoplasms in patients treated with infliximab did not exceed the reported incidence in the general population.
- Cardiovascular failure. Cases of progression of cardiovascular failure during the use of infliximab have been described. There are rare reports of newly diagnosed cardiovascular failure, including in patients who had no previous diseases of the cardiovascular system.
- Changes in the liver and biliary tract. There are very rare reports of the appearance of jaundice and non-infectious hepatitis, in some cases having signs of autoimmune hepatitis, the development of liver failure, leading to the need for a liver transplant or fatal outcome. A cause-and-effect relationship between the occurrence of these adverse reactions and treatment with infliximab has not been established. There have been cases of exacerbation of hepatitis B in patients who were chronic virus carriers (who had a positive reaction to HBsAg).
- When treated with infliximab, a mild to moderate increase in aminotransferase activity may be observed without the development of significant liver damage. In most cases, the increase in aminotransferase levels is transient and asymptomatic. A decrease or return to the initial level of these indicators occurs regardless of whether treatment with infliximab is continued or stopped or concomitant therapy is changed. An increase in alanine aminotransferase activity to a level equal to or greater than 5 times the upper limit of normal was observed in 1% of patients.
- When using infliximab, isolated cases of the development of demyelinating diseases of the central nervous system, optic neuritis, and epileptic seizures have been described.
- In some patients, antinuclear antibodies appeared in the blood serum during treatment with infliximab. Cases of reversible lupus-like syndrome have been described.
Pregnancy/lactation
Infliximab is not recommended for use during pregnancy. During treatment with the drug and for 6 months. after its completion, reliable methods of contraception should be used. Breastfeeding should be discontinued when infliximab is prescribed. Breastfeeding is allowed no earlier than after 6 months. after the end of therapy.
Prevention and treatment of adverse reactions
For early detection of an acute infusion reaction, the patient should be carefully observed during and for at least 1-2 hours after the drug infusion. If an acute infusion reaction occurs, the drug should be discontinued. When conducting infliximab infusions, it is necessary to have appropriate equipment and medications (adrenaline, glucocorticosteroids for parenteral administration, antihistamines, equipment for artificial ventilation). To prevent mild and transient infusion reactions, the patient may be prescribed antihistamines before starting the infusion.
Contraindications/restrictions
- Hypersensitivity reactions to infliximab, other murine proteins, as well as to any of the inactive components of the drug.
- Severe infectious process, for example, sepsis, abscess, tuberculosis or other opportunistic infection.
- Heart failure - severe or moderate.
- Pregnancy and breastfeeding.
- Age less than 18 years.
Interaction
The interaction of infliximab with other drugs has not been studied. Concomitant use with methotrexate in patients reduces the formation of antibodies to infliximab and increases its concentration in plasma.
Overdose
A single administration of infliximab in doses up to 20 mg/kg did not cause direct toxic effects. In case of overdose, observation and immediate relief of symptoms are necessary.
special instructions
- Infliximab, when administered, can cause the development of acute allergic reactions (immediate type) and delayed allergic reactions. Acute infusion reactions may develop immediately or within several hours after administration. A patient receiving infliximab should be monitored during and for at least 1-2 hours after the drug infusion.
- Some patients may develop antibodies to infliximab, which is associated with a more frequent development of infusion reactions. In patients suffering from Crohn's disease, there has been a correlation between the formation of antibodies and a decrease in the duration of the effect of treatment. Patients who stop taking immunosuppressive drugs before or during treatment with infliximab are more at risk of developing these antibodies. If severe reactions develop, symptomatic therapy should be carried out, and further use of the drug should be excluded.
- As the interval between infusions increases, the likelihood of the formation of antibodies to infliximab increases. When re-prescribing infliximab after a long break in treatment, it is necessary to be wary of the appearance of a delayed-type hypersensitivity reaction in the patient.
- When prescribing infliximab to patients with a history of indications of malignant neoplasms, or when deciding whether to continue treatment with infliximab in patients with newly diagnosed neoplasms, special caution should be exercised.
- Before starting treatment with infliximab, the patient should be carefully examined to identify both active and latent tuberculosis. The examination should include:
- careful collection of anamnesis (presence of tuberculosis in the past and/or contact with tuberculosis patients);
- X-ray examination of the chest in two projections;
- conducting a tuberculin test;
- consultation with a phthisiatrician.
- If a tuberculous process is suspected, the use of infliximab should be discontinued until diagnosis is made and, if necessary, appropriate therapy should be administered.
- The patient should be informed that he needs to consult a doctor if the following symptoms appear during treatment with infliximab or after its completion: cough, weight loss, low-grade body temperature.
- During treatment and after its completion, the patient should be closely monitored for signs of possible infection. Since elimination of infliximab occurs within 6 months, the patient should be constantly under medical supervision during this period. Treatment with infliximab should be discontinued if the patient develops a severe infection, including tuberculosis, sepsis or pneumonia.
- The use of live vaccines during treatment with infliximab is not recommended.
- In rare cases, an autoimmune process may develop in genetically predisposed patients. In the event of persistent rash, fever, joint pain, fatigue, or the presence of DNA antibodies in the blood, treatment with infliximab should be discontinued.
- The benefits and risks of infliximab should be carefully weighed in patients with pre-existing or new-onset CNS demyelinating disease.
- Patients with moderate circulatory insufficiency should be carefully monitored. If symptoms of circulatory failure increase, infliximab should be discontinued.
- Patients with signs of liver dysfunction should be evaluated for liver disease. If jaundice occurs or alanine aminotransferase activity increases to levels greater than 5 times the upper normal value, infliximab should be discontinued and a thorough evaluation should be performed.
- Hepatitis B virus carriers should be examined before treatment with infliximab and continuously monitored during treatment in order to timely detect a possible exacerbation of the disease.
- The effectiveness and safety of treatment with infliximab in children and adolescents under the age of 18 years, inclusive, suffering from psoriatic arthritis and psoriasis have not been studied. Until convincing data are obtained, the drug should not be used in these age groups.
- There have been no specific studies on the use of infliximab in elderly people, as well as in people with liver and kidney diseases. There is limited experience indicating the safety of treatment with infliximab in patients undergoing arthroplasty.
Monitoring laboratory parameters during treatment with infliximab
| Infusion weeks | ||||
| Before | 2nd week | 6th week | Every 8 weeks | |
| General blood test1 | X | X | X | X |
| General urine analysis | X | X | X | X |
| ALT,AST | X | X | X | X |
| Pregnancy test | X | |||
Note. 1 Hemoglobin, hematocrit index, erythrocytes, leukocytes, leukocyte formula, platelets.
2. Adalimumab
The selective immunosuppressive drug adalimumab is a completely identical human monoclonal antibody that blocks the activity of TNF-a, a pro-inflammatory cytokine that plays a key role in the pathogenesis of psoriasis. Doses and regimen. For chronic plaque psoriasis, the initial dose for adult patients is 80 mg. Maintenance dose - 40 mg once every 2 weeks, starting a week after the initial dose. The drug is administered subcutaneously into the thigh or abdomen.
Overdose.
The maximum tolerated dose of adalimumab in humans has not been established. Repeated use of adalimumab in doses up to 10 mg/kg was not accompanied by toxic effects requiring dose reduction. In case of overdose, it is necessary to monitor adverse reactions and immediately begin adequate symptomatic treatment.
Drug interactions
In patients with rheumatoid arthritis receiving methotrexate, there is no need for dose adjustment of adalimumab or methotrexate. However, methotrexate with single and repeated use reduces the clearance of adalimumab by 29% and 44%, respectively. The interaction of adalimumab with other drugs other than methotrexate has not been studied in pharmacokinetic studies. In clinical studies, there were no signs of interaction of adalimumab with other basic drugs (sulfasalazine, hydrochloroquine, leflunomide and parenteral gold preparations), corticosteroids, salicylates, NSAIDs and analgesics.
Pregnancy and lactation.
Adalimumab is contraindicated during pregnancy and breastfeeding. Adequate and strictly controlled studies of the drug in pregnant women have not been conducted. Women of reproductive age should avoid conceiving while being treated with the drug. Given the risk of serious side effects in the newborn, it is advisable to stop breastfeeding or discontinue the drug, taking into account its importance for the mother.
Side effects
- According to clinical studies, approximately 15% of patients can be expected to develop injection site reactions, which are one of the most common side effects with adalimumab in controlled clinical trials.
- Infections: very often - respiratory tract infections (including upper and lower respiratory tract infections, pneumonia, sinusitis, pharyngitis, nasopharyngitis and herpes viral pneumonia); often - generalized infections (including sepsis, candidiasis and influenza), gastrointestinal infections (including viral gastroenteritis), skin and soft tissue infections (including paronychia, cellulitis, impetigo, necrotizing fasciitis and herpes zoster), ear infections, oral infections (including herpes simplex, oral herpes and dental lesions), genital infections (including vulvovaginal mycotic infection), urinary tract infections (including pyelonephritis); uncommon - opportunistic infections and tuberculosis (including coccidioidomycosis, histoplasmosis and complex of infections caused by Mycobacterium avium), neurological infections (including viral meningitis), eye infections, bacterial infections, joint infections.
- Neoplasms: often - benign neoplasms, skin cancer, except melanoma (including basal cell carcinoma and squamous cell carcinoma); uncommon - lymphoma, parenchymal neoplasms, neoplasms of the mammary gland, lungs and thyroid gland, melanoma.
- From the blood and lymphatic system: very often - leukopenia (including neutropenia and agranulocytosis), anemia; often - thrombocytopenia, leukocytosis; uncommon - idiopathic thrombocytopenic purpura; rarely - pancytopenia.
- From the immune system: often - hypersensitivity reactions, seasonal allergies.
- Metabolism: very often - increased lipid levels; often - hypokalemia, increased uric acid levels, pathological changes in sodium content, hypocalcemia, hyperglycemia, hypophosphatemia, increased potassium levels in the blood; infrequently - dehydration.
- From the nervous system: very often - headache; often - paresthesia (including hypesthesia), migraine, sciatic neuralgia, mood changes (including depression), irritability, insomnia, dizziness; infrequently - tremor; rarely - multiple sclerosis.
- From the senses: often - conjunctivitis, visual impairment; infrequently - blepharitis, eyelid edema, diplopia, deafness, ringing in the ears.
- From the cardiovascular system: often - arterial hypertension, hot flashes, hematomas, tachycardia; uncommon - arrhythmia, congestive heart failure; rarely - cardiac arrest, arterial occlusion, thrombophlebitis, aortic aneurysm.
- From the respiratory system: often - cough, asthma, dyspnea; infrequently - chronic obstructive pulmonary disease, interstitial lung diseases.
- From the digestive system: very often - nausea, vomiting, abdominal pain, increased activity of liver enzymes; often - dyspepsia, gastroesophageal reflux, dry mouth (sicca syndrome), gastrointestinal bleeding; uncommon - pancreatitis, dysphagia, facial edema, cholecystitis, cholestasis, increased bilirubin levels, hepatic steatosis.
- Dermatological reactions: very often - rash (including exfoliative); often - itching, urticaria, hemorrhages (including purpura), dermatitis and eczema, brittle nails, hyperhidrosis; Uncommon: night sweats, scars.
- From the musculoskeletal system: very often - musculoskeletal pain; often - muscle spasms; uncommon - rhabdomyolysis; rarely - systemic lupus erythematosus.
- From the urinary system: often - hematuria, renal failure; infrequently - nocturia.
- From the reproductive system: infrequently - nocturia, erectile dysfunction.
- From laboratory indicators: often - violations of indicators
- blood clotting (including increased aPTT), positive tests for autoantibodies (including antibodies to the double helix of DNA), increased LDH levels.
- Local reactions: very often - reactions at the injection site (including erythema).
- Other: often - chest pain, swelling; infrequently - inflammation, deterioration of wound healing.
special instructions
.
Adalimumab has a number of important features. In particular, the drug has no risk of infusion reactions, since adalimumab is administered subcutaneously. The frequency of formation of antibodies to adalimumab is only 1.9-8.4%, which undoubtedly has a beneficial effect on both the safety profile of long-term use of the drug and has a positive effect on the level and duration of clinical response to treatment.
Long-term follow-up of psoriasis patients treated with open-label adalimumab over 3 years in the large REVEAL study is available. The effectiveness and safety of continuous therapy with adalimumab in patients with moderate to severe psoriasis for more than three years was assessed. The studies demonstrated that in patients with clinical responses to treatment PASI 75, PASI 90 and PASI 100, reflecting a decrease in the extent and severity of the skin process by 75%, 90%, 100%, respectively, the effectiveness of adalimumab was maintained for three years. Thus, after 160 weeks of continuous treatment with this biological agent, the percentage of patients with PASI 75 remained at 76%; PASI 90 - respectively 50%; PASI 100 - 31%. The data obtained suggest that the majority of patients with psoriasis who received adalimumab responded well to treatment carried out for more than 3 years. More than half of the observed individuals after 160 weeks of treatment with adalimumab had only minimal manifestations of psoriasis on the skin, and in one third of the patients the psoriatic process resolved completely. The most stable response to long-term treatment with adalimumab was found in patients with an improvement in PASI 100. The study made it possible to significantly clarify the data on a possible decrease in the effectiveness of the biological agent with long-term use. It was found that it is extremely important to assess response early in treatment with adalimumab. A good initial response rate with adalimumab (PASI 75 or more at weeks 16 and 33) is, in fact, a predictor of favorable long-term treatment outcomes over a follow-up period of 3 years or more. Finally, the study demonstrated the high safety of long-term use of adalimumab. It is important that among all study participants (n=1159), only 2 cases of tuberculosis were registered. Not a single case of lymphoma, lupus-like syndrome, or demyelinating disease was identified.
According to an analysis of the safety of adalimumab in psoriasis, where the assessment was based on data from 13 clinical studies, and the duration of treatment with the drug was up to 5 years, the frequency (expressed as the number of events per 100 PL) of serious AEs, severe infections, malignant neoplasms does not increase or even decreases as increasing the duration of treatment.
3. Ustekinumab
Ustekinumab is a fully human IgG1k monoclonal antibody that has high affinity and specificity for the p40 subunit of human interleukins (IL) IL-12 and IL-23. Ustekinumab is indicated for the treatment of patients over 18 years of age with moderate to severe plaque psoriasis, as well as patients with active psoriatic arthritis as monotherapy or in combination with methotrexate.
Directions for use and doses
Ustekinumab is intended for subcutaneous injection. The recommended dose is 45 mg. The second injection is given 4 weeks after the first application, then every 12 weeks. In patients weighing more than 100 kg, the drug is recommended to be used at a dose of 90 mg. For patients in whom the clinical effectiveness of the drug when used every 12 weeks is not sufficiently expressed, the dose of the drug should be increased to 90 mg every 12 weeks. If this dosing regimen is not effective, a dose of 90 mg should be administered every 8 weeks. Resumption of therapy according to the proposed regimen - a second injection 4 weeks after the first use, and then every 12 weeks - was as effective as the first therapy .
Side effects
The following side effects were observed in clinical studies of ustekinumab:
- infections (odontogenic infections, upper respiratory tract infections, nasopharyngitis, inflammation of subcutaneous fat, herpes zoster, viral infections of the upper respiratory tract);
- mental disorders (depression);
- from the central nervous system (dizziness, headache);
- from the respiratory system (pain in the throat and larynx, nasal congestion);
- from the gastrointestinal tract (diarrhea, vomiting);
- from the skin and subcutaneous tissues (itching);
- from the musculoskeletal system (back pain, myalgia, arthralgia);
- general disorders and reactions at the injection site (fatigue, erythema at the injection site, pain at the injection site, reactions at the injection site, including hemorrhage, hematoma, induration, swelling and itching);
- malignant neoplasms (non-melanoma skin cancer, malignant neoplasms of the prostate, intestines, mammary glands and melanoma in situ);
- hypersensitivity reactions (rash, urticaria).
Immunogenicity: Approximately 6% of patients receiving ustekinumab developed antibodies to the drug, which usually had a low titer. There was no clear correlation between the formation of antibodies and the presence of reactions with the injection. Most patients who had antibodies to ustekinumab also had antibodies that neutralized them. In the presence of antibodies to ustekinumab, patients often had lower efficacy of the drug, although the presence of antibodies does not exclude the achievement of a clinical effect.
In post-registration use of ustekinumab, adverse events from the immune system were identified: hypersensitivity reactions (including rash and urticaria), serious hypersensitivity reactions (including anaphylaxis and angioedema); from the skin and subcutaneous tissues: plaque psoriasis.
Pregnancy and lactation
It is not recommended to use the drug during pregnancy; effective methods of contraception should be used during and 15 weeks after treatment with the drug.
A decision should be made to stop breastfeeding while taking the drug or to discontinue ustekinumab therapy.
Contraindications
- Clinically significant hypersensitivity to ustekinumab or any excipient of the drug;
- Children's age (up to 12 years);
- Pregnancy and lactation;
- Serious infectious diseases in the acute phase, including tuberculosis;
- Malignant neoplasms.
Carefully
- Chronic or recurrent parasitic and infectious diseases of a viral, fungal or bacterial nature.
- History of malignant neoplasms.
- Elderly age.
Overdose
During clinical studies, patients were given single intravenous doses of up to 6 mg/kg without the development of dose-limiting toxicity. In case of overdose, it is recommended to monitor the patient's condition for signs and symptoms of side effects and, if they develop, appropriate symptomatic therapy should be started immediately.
special instructions
- Ustekinumab is a selective immunosuppressant and may increase the risk of developing infections and reactivation of latent infections. In clinical studies, serious bacterial, fungal and viral infections were observed in patients using ustekinumab. Ustekinumab should not be used in patients with clinically significant, active infections. Caution should be exercised when using the drug in patients with chronic infections or a history of recurrent infections.
- Before starting to use the drug, the patient should be tested for the presence of tuberculosis. Ustekinumab should not be used in patients with active tuberculosis. If you have latent or active tuberculosis (including a history of tuberculosis), treatment should be started before using ustekinumab. Treatment of tuberculosis should also be started in patients in whom the sufficient effect of previous treatment is unconfirmed. During and after treatment with ustekinumab, patients should be closely monitored for signs and symptoms of active tuberculosis.
- Patients should be warned to seek medical attention if signs and symptoms suggestive of infection appear. If a serious infection develops, the use of ustekinumab should be discontinued and the patient should be under the supervision of medical personnel. Ustekinumab should not be used until treatment for the infection has been completed.
- Immunosuppressants may increase the risk of developing malignancies. In some patients receiving ustekinumab in clinical trials, the occurrence of malignant neoplasms (cutaneous and non-cutaneous forms) was observed. The use of ustekinumab has not been studied in patients with a history of malignancy. Caution should be exercised when prescribing the drug to patients with a history of malignancy, as well as when considering continued treatment with ustekinumab in patients diagnosed with malignancy. All patients over the age of 60 years, as well as those who have previously received long-term therapy with immunosuppressants or UV radiation, should be screened for the presence of non-melanoma skin cancer.
- Serious hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during post-marketing use of ustekinumab. If anaphylactic or other serious hypersensitivity reactions occur, ustekinumab should be discontinued immediately and appropriate treatment should be instituted.
- The patient should not be vaccinated with live vaccines during the period of treatment with ustekinumab, as well as in the period 15 weeks before vaccination (after taking the last dose of the drug) and 2 weeks after vaccination. Caution should be exercised when using live vaccines to immunize family members of a patient receiving ustekinumab, since there is a risk of viral or bacterial release and transmission of infection from these individuals to patients. Long-term treatment with ustekinumab does not suppress the humoral immune response to vaccines containing pneumococcal polysaccharide and tetanus vaccine. Vaccines containing inactivated microorganisms can be used with ustekinumab, but the induced immune response may not be sufficient to prevent disease.
- Concomitant immunosuppressive therapy: The safety and effectiveness of ustekinumab in combination with immunosuppressive drugs and phototherapy have not been studied in patients with psoriasis. In studies in patients with psoriatic arthritis, coadministration with methotrexate did not affect the safety and efficacy of ustekinumab. Caution should be exercised when considering the possibility of simultaneous
- the use of other immunosuppressants and ustekinumab, as well as when switching from therapy with another antipsoriasis biological drug to ustekinumab therapy.
- Use in elderly patients (over 65 years): Of the 4031 patients treated with ustekinumab, 248 were patients over 65 years of age (183 patients with psoriasis and 65 with psoriatic arthritis). In clinical studies, there was no effect of age on clearance or volume of distribution of the drug. Although studies of the drug showed no differences in the safety and effectiveness of the drug in elderly patients over 65 years of age compared with younger patients, the number of elderly patients is insufficient to make a definitive conclusion about the effect of age (or lack thereof) on clinical effectiveness.
Use in children: The safety and effectiveness of ustekinumab in children has not been studied. The drug has not been studied in patients with renal or hepatic impairment.
Special instructions.
Currently, there is data on the effectiveness and safety of continuous therapy with ustekinumab for 5 years in patients with moderate to severe psoriasis. Multicenter, double-blind, randomized, placebo-controlled studies have shown that the effectiveness of ustekinumab remains high with continuous use of the drug for 5 years, and the most common adverse events are upper respiratory tract infections.