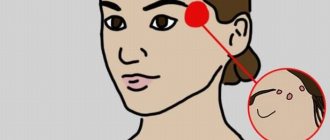
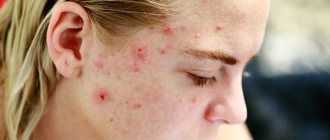
Pimples on the scalp in the hair are a very common occurrence. Unfortunately, it is usually not given importance, since the rashes are hidden behind the strands. As a rule, the patient turns to a specialist only when the problem becomes widespread. However, it should be remembered that treatment in this case turns out to be much more complex and lengthy.
Why do acne appear on the head?
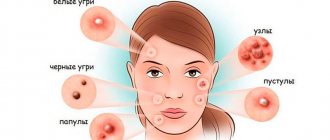
The causes of acne on the head may be the following:
- disruption of the sebaceous glands;
- poor nutrition;
- insufficient or improper hair or skin care, as well as the wrong choice of care products;
- hormonal disturbances or physiological changes (for example, during puberty);
- increased sweating;
- folliculitis (its development may be indicated by purulent pimples on the head);
- constant wearing of hats made of synthetic materials;
- allergic reactions;
- frequent scratching, traumatic effects;
- diseases of the nervous system;
- dysfunction of the adrenal glands;
- decreased immunity;
- autoimmune disorders;
- hereditary predisposition;
- infectious diseases;
- taking certain medications (for example, corticosteroids).
Pimples on the scalp in men are most often caused by hormonal activity, stress, exposure to chemicals (for example, during industrial work), and skin reactions after shaving. In addition, breakouts can be a result of using dull trimmer blades while trimming. Acne on the head of women sometimes appears when using oral contraceptives.
Also, among other reasons, they can signal ovarian diseases, in particular polycystic disease. That is why, if the phenomenon is recurrent, a consultation with a gynecologist may be required.
How to get rid of it?
To completely eliminate unpleasant skin rashes, it is necessary to find out and eliminate the causes that led to their appearance.
When dealing with acne, it is very important to adhere to the following principles:
- Maintain proper, healthy nutrition. Eat as little fatty, sweet foods and foods that contain large amounts of simple carbohydrates as possible.
- Maintain personal hygiene. It is necessary to ensure that the surface of the skin of the face and hands is clean; be sure to wash your face in the morning and evening hours. However, it is worth remembering that too frequent water treatments can dry out the skin and weaken its protective function.
- Regularly use antiseptic creams.
- Do not squeeze pimples. Such actions are fraught with the spread of infection into the deep layers of the epidermis and aggravation of the inflammatory process.
- Do not cleanse your skin with alcohol lotions. These cosmetics irritate the sebaceous glands and increase their secretion.
- Do not expose the skin to the active influence of solar radiation, as it increases the production of sebum.
If acne was caused not just by poor diet or lack of personal hygiene, but by hormonal imbalance or disorders of the internal organs, then just following these rules will not be enough for recovery. In such cases, specialists often prescribe drugs with local or even systemic action.
On the face
If the occurrence of acne is associated with hormonal imbalance, for mild acne it is enough to use topical symptomatic medications.
The most popular are:
- Skinoren gel.
- Differin gel.
- Baziron gel.
- Curiosin gel.
Heavy rashes on the skin can be dried using simple tar soap, as well as calendula tincture. For disinfecting, cleansing and preventing inflammation, local antiseptics are used - Ichthyol ointment, Metrogyl gel.
In cases where acne has 3-4 degrees of severity, systemic therapy is prescribed, which consists of using antibacterial (Bactroban ointment, Fusiderm cream, Zinerit in powder form, Levomikol ointment) and hormonal agents (Hydrocortisone ointment and its analogues).
They should be used carefully, according to the instructions and only as prescribed by the attending physician.
On the body
Acne in men can appear not only on the face, but also on various parts of the body - back, chest, arms. For mild acne, it is enough to adhere to a healthy diet, good personal hygiene and treat the skin surface with products that contain salicylic acid or benzoyl peroxide, as well as cosmetics based on glycolic or lactic acids.
For moderate to severe acne, the help of a specialist is necessary. In severe cases, Isotretinoin may be prescribed. The drug has a large number of side effects, so using it on your own is strictly prohibited. Other retinoids and antibiotics may also be prescribed.
Treatment of acne on the head
The course of therapy depends on the causes of the problem, however, as a rule, it includes:
- antimicrobials;
- enterosorbents;
- retinoids;
- means that dry out rashes.
In addition, clinical procedures such as mesotherapy, laser treatment, biocomplex, and plasma therapy are effective in this case.
When the rash spreads severely, it is sometimes necessary to take antibacterial drugs, as well as drugs to correct hormonal levels. To prevent the recurrence of acne on the scalp, the patient is advised to choose the right hygiene products, normalize nutrition, monitor the hormonal status of the body, take precautions when interacting with chemicals, take vitamins and microelements, and give up bad habits.
Skin manifestations of pathology of the digestive organs
The skin and digestive organs are in close relationship, the roots of which lie in the same origin during embryogenesis, common barrier functions, including the formation of an immune response, as well as interaction with the microflora associated with these organs. The condition of the skin largely reflects the condition of the digestive organs, however, the relationship between pathological processes in these organs can be diverse: 1) skin damage can be directly caused by dysfunction of the digestive organs; 2) skin lesions may have a common pathogenesis with diseases of the digestive organs, precisely established or suspected, and these processes occur in parallel.
The idea that dysfunction of the digestive organs naturally affects the condition of the skin, although it has been in the air for thousands of years, was first formulated in the medical literature by William Hillary in 1759 [1] and developed by A. Whitfield. The latter introduced the concept of “dermatitis colonica” in 1932 [2], and one of the first detailed reviews on the effect of impaired intestinal absorption on the skin condition was published by GC Wells only in 1962 [3].
Malabsorption of macro- and micronutrients with the development of their deficiency in the body in various diseases of the gastrointestinal tract (GIT) is obviously reflected in the condition of the skin. Most often they are observed with skin changes due to impaired intestinal absorption, which is caused by malabsorption of zinc, copper, iron, vitamins A and PP. The development of purpura can also be included in the same category as a manifestation of hemocoagulopathy due to impaired absorption of vitamin K. Selective deficiency of a particular nutrient is usually associated with a congenital defect of the corresponding intestinal transport system, while combined disorders of intestinal absorption are more often observed with generalized intestinal damage (congenital or acquired), for example, with celiac disease or short small bowel syndrome. The nature of skin damage due to deficiency of certain vitamins is given in Table. 14].
A striking clinical manifestation of the skin is impaired intestinal absorption of zinc. In general, the causes of zinc deficiency in the body can be its lack of nutrition (for example, with irrational artificial feeding), various intestinal diseases, especially those accompanied by diarrhea, as well as a congenital defect in its intestinal absorption (acrodermatitis enteropathica).
The absorption of zinc in the intestine is closely related to the nature of motility and easily develops in the case of diarrhea of any origin. Thus, it has been shown that diarrhea in children under 1 year of age lasting more than 10 days is always accompanied by the development of zinc deficiency [5], and in older children, diarrhea lasting more than 14 days can lead to this condition [6]. At the same time, accelerated intestinal motility, disrupting the absorption of zinc, contributes to the loss of zinc entering the intestinal lumen with the secretions of the digestive glands. In addition, zinc deficiency leads to dysfunction of enterocytes, which in turn aggravates its malabsorption. A decrease in immune functions due to zinc deficiency favors the course of the infectious process and also aggravates diarrhea [7]. Zinc deficiency and impaired enterocyte function contribute to malabsorption of other nutrients, particularly sodium and water [8]. It is also interesting that zinc deficiency leads to a decrease in appetite as a result of a decrease in the expression of neuropeptide Y receptors in the hypothalamus and thereby contributes to a decrease in food intake [9].
Acrodermatitis enteropathica (Brandt syndrome) is characterized by a congenital disorder of zinc transport across the apical membrane of the enterocyte. The disease is transmitted in an autosomal recessive manner and is clinically manifested by a characteristic skin syndrome (skin lesions in the form of vesiculobullous rashes on the distal parts of the limbs and around natural openings), diarrhea and alopecia. The disease manifests itself after stopping breastfeeding, since human milk is an important source of zinc for the child during this period of life, with the gradual development of the main symptoms of the disease, delayed psychomotor development, and a decrease in resistance to infectious diseases. The latter is due to the significant role played by zinc in the function of the immune system, antioxidant protection, and even some direct anti-infective effects of this microelement. A condition similar to enteropathic acrodermatitis can also develop secondary to severe atrophy of the mucous membrane of the small intestine, primarily with celiac disease. In both cases, eliminating zinc deficiency requires the administration of drugs containing it.
Another selective defect is observed in Menkes disease, which is based on a violation of the cellular transport of copper in the intestine. In this case, there is a slowdown in growth, a pathology of the nervous system, as well as a characteristic curling of the hair, which is why this disease is called “curly or steel hair disease.” The disease is associated with mutations in the ATP7A gene, which encodes a Cu(2+)-transporting ATPase involved in the processes of intestinal absorption of copper.
The disease manifests itself in the neonatal period, and the first symptoms may be: hypothermia, hyperbilirubinemia, and delayed physical development. Some patients experience the formation of hair nodules (trichorexis nodosa), etc. spindle-shaped hair (monilethrix). In addition, seborrheic dermatitis may occur. During the first half of life, growth retardation, delayed psychomotor development and neurological disorders gradually progress with the loss of skills acquired in the first months of life, various types of epileptic seizures appear (focal, generalized, myoclonic) with the subsequent formation of spastic tetraparesis. Trichopolydystrophy develops: hair is matted, dull, hard, gray or ivory-colored.
The development of subdural hemorrhages and thrombotic disease is possible. Angiography in the brain, internal organs, and limbs reveals elongated, tortuous, varying-caliber arteries with alternating areas of expansion and narrowing. In addition, urinary tract diverticula may be identified, predisposing to urinary tract infections. Pathological fractures of the ribs, diffuse hypopigmentation of the skin, as well as signs of connective tissue dysplasia: hyperextensibility of the skin, hypermobility in the joints are often observed.
The diagnosis is made on the basis of a decrease in the level of copper and ceruloplasmin in the blood serum, low copper content in the liver biopsy and high copper in the intestinal mucosa and cultured skin fibroblast cells. Treatment is based on copper replacement therapy.
The following are skin diseases described in combination with celiac disease, a disease with generalized intestinal malabsorption (Table 2) [10, 11]. Some of them are due to malabsorption of macro- and micronutrients, another part is due to increased intestinal permeability against the background of the atrophic process and an increased risk of an allergic process, and the third part is due to an autoimmune process in the skin, which can be considered as a manifestation of the underlying pathological process. Dermatitis herpetiformis requires special discussion, which has much in common with celiac disease, but also has significant differences.
General mechanisms of damage to the skin and digestive organs are also observed quite often, for example, in various immunopathological processes. At the same time, dysfunction of the gastrointestinal tract and, in particular, malabsorption syndrome aggravate the course of the skin process with the participation of the mechanisms described above.
In food allergies, as a rule, several body systems are simultaneously involved: skin (atopic dermatitis), gastrointestinal tract (eosinophilic esophagitis, gastritis, enteritis, colitis), respiratory organs (allergic rhinitis, bronchial asthma) [12]. In recent years, food allergies have been given importance in the development of irritable bowel syndrome. One of the points of intersection of these diseases is visceral reception, which, on the one hand, is modulated by central nervous influences, and on the other hand, by the so-called. inflammation of low activity in the intestinal mucosa, the main role in the development of which is played by degranulation of mast cells. The latter can be caused by various mechanisms, including atopic ones [13].
In systemic autoimmune connective tissue diseases, combined damage to the skin and gastrointestinal tract is often observed. Thus, with systemic scleroderma, characteristic skin changes are combined with impaired motility of the esophagus and dysphagia, impaired motility of the small intestine and intestinal obstruction of varying severity, as well as the syndrome of impaired intestinal absorption [14–18]. With systemic vasculitis and rheumatoid arthritis, parallel damage to the arteries in various organs and systems occurs, but subsequently the malabsorption caused by this process aggravates skin changes.
Inflammatory autoimmune bowel diseases, ulcerative colitis and Crohn's disease are also characterized by skin lesions in some patients. They are often associated with erythema nodosum and pyoderma gangrenosum, which have the same pathogenesis as the main disease and are observed in approximately 3–12% of patients [19–21]. Erythema nodosum manifests itself as painless, red, subcutaneous nodules on the extensor surfaces of the extremities, and its activity corresponds to the activity of the underlying disease [22]. Biopsy reveals focal panniculitis. Erythema nodosum responds well to treatment with glucocorticoid drugs, and in resistant cases with infliximab [23, 24]. Pyoderma gangrenosum is characterized by severe ulceration, lack of parallelism with the severity of the disease and poses a serious challenge in terms of treatment therapy. A biopsy reveals a sterile abscess. Treatment requires the use of high doses of glucocorticoids, as well as cyclosporine, tacrolimus, mycofenrolate mofetil, and infliximab [23–26].
The development of erythema nodosum in Crohn's disease is due to an underlying granulomatous inflammatory process, and the development of severe pyoderma gangrenosum is due to inadequate antimicrobial protection, perhaps partly related to a decrease in the production of defensins in the skin (as is observed in the intestines).
Other cutaneous manifestations of inflammatory bowel disease include aphthous stomatitis, which usually responds well to steroid medications [27, 28].
A separate group consists of diseases in which persistent associations between intestinal pathology and changes in the skin are observed, although the mechanism of this association remains unidentified. An example of such a disease is Peutz-Jeghers syndrome.
This disease was first described by the Danish pediatrician Y. Peutz in 1921. Under his supervision there was a family whose members had a combination of skin pigmentation with polyposis of the small intestine. A similar observation was presented in 1949 by H. Jeghers [29].
Peutz-Jeghers syndrome is a congenital disease, transmitted in an autosomal dominant manner, characterized by pigmentation of the skin and mucous membranes in combination with polyps of the gastrointestinal tract. Pigment spots with a diameter of 1–5 mm usually appear in the first years of life, although they are also described in newborns, located around the mouth, nasal openings, on the buccal mucosa, on the hands and feet. Perianal and perigenital localization is less common. Interestingly, spots located around the mouth and nose do not disappear over time, while the severity of spots in other locations may decrease during puberty. Polyps are most often detected in the jejunum, but are also found in the stomach, duodenum, and colon. Polyps can cause intestinal obstruction, bleeding, and they can also become malignant. The prevalence of the syndrome is 1:120,000 live births [30], with no sex differences [31]. Its development is associated with a mutation in the STK11 (LKB1) gene localized on chromosome 19 p13.3 [32].
The STK11 gene encodes serine-threonine kinase, a modulator of cell proliferation that plays a significant role in preventing the development of tumors [33]. In this regard, the enzyme encoded by STK11 inhibits AMP-activated protein kinase and, indirectly, the mTOR signaling pathway (mammalian target of rapamycin) [34], disturbances of which have been identified in patients with Peutz-Jeghers syndrome [35].
Pigment spots are caused by an increased number of melanocytes in the area of the dermoepidermal junction (dermoepidermal junction) with an increase in the content of melanin in the basal cells. Polyps of the gastrointestinal tract are hamartomas, consisting of normal tissue of an organ, but with a disturbed architecture. Polyps in this syndrome consist of glandular epithelium and smooth muscle tissue connecting to the muscularis mucosae (muscular plate of connective tissue).
Polyps are always multiple. Localized in the small intestine (64%), less often in the large intestine (53%), stomach (49%) and rectum (32%). Typically, fewer than 20 polyps are found in each case, and the size ranges from a few millimeters to 5 cm in diameter [36].
In addition to Peutz-Jeghers syndrome, hamartomatous polyps are also observed in juvenile polyposis and hamartoma tumor syndrome associated with the PTEN gene (PTEN hamartoma tumor syndrome). The latter include Cowden syndrome, Bannayan–Riley–Ruvalcaba syndrome and others caused by a mutation of this gene.
Differential diagnosis of pigmented lesions is carried out with LEOPARD, Langier–Hunziker, Carney, Cowden syndromes and normal skin condition observed in some African Americans [37, 38].
Of particular interest is the analysis of the relationship between Dühring's dermatitis herpetiformis and celiac disease. On the one hand, the pathogenesis of both diseases lies in intolerance to the cereal protein gluten with the development of atrophy of the mucous membrane of the small intestine and, as a consequence, a generalized violation of intestinal absorption. The latter is most pronounced in celiac disease, although there are a significant number of atypical forms in which the intestinal syndrome fades into the background. Atypical celiac disease often presents with iron deficiency anemia, or osteoporosis, or short stature, as well as many other extraintestinal symptoms. Dühring's dermatitis herpetiformis is not an atypical form of celiac disease, but an independent disease in which gluten-sensitive individuals develop characteristic skin lesions.
In both celiac disease and dermatitis herpetiformis, a characteristic HLA haplotype, circulating antibodies to tissue transglutaminase, and villous atrophy of the small intestinal mucosa can be identified, with a positive effect on its status of a gluten-free diet. It should be noted, however, that in approximately 20% of patients with dermatitis herpetiformis, the condition of the small intestinal mucosa remains normal [39, 40].
Clinically, dermatitis herpetiformis manifests itself as herpetiform grouped polymorphic elements (vesicles, blisters, pustules, papules and papulovesicles) on unchanged or swollen erythematous areas of the skin, appearing simultaneously or at short intervals. The bladder cover is dense. The contents of the bubble are initially transparent, then cloudy. Blisters can turn into pustules, open with the formation of erosions, on the surface of which crusts form, followed by epithelization. Blisters may appear along the periphery of such areas. The rash is usually located symmetrically and is grouped in the form of rings, half rings or garlands (herpetiform grouping). Typical sites of localization are the extensor surfaces of the limbs and the buttocks. The appearance of a rash is often preceded or accompanied by general malaise, headaches, insomnia, dyspeptic symptoms, and an increase in temperature from 37.5 to 40 °C. The itching is usually severe, and sometimes a burning sensation is possible. The exacerbation of the disease continues for several weeks, and in some areas the rash resolves, passing through the stage of secondary elements (erosion, scales, crusts, hyper- or hypopigmentation), while in others fresh rashes appear. There are no rashes on the scalp. In children, the mucous membranes are quite often affected [41], and there is an increased tendency to form pustules.
The pathogenesis of both diseases remains completely unsolved. At the same time, the nature of the pathological process in the skin differs from that in the small intestine and is characterized by the presence of an inflammatory infiltrate, predominantly consisting of neutrophils, localized in the dermal papillae, where IgA deposits are also found [42]. The importance of neutrophils in the pathological process is confirmed by the positive effect on skin changes of the administration of dapsone, which suppresses the activity of neutrophils. This is also indicated by increased expression of CD11b, a slight decrease in L-selectin, and increased function of FcIgA receptors [43]. At the same time, in the small intestine, both in celiac disease and in dermatitis herpetiformis, a predominantly mononuclear reaction is observed.
It is also noteworthy that a gluten-free diet, effective for celiac disease, is not effective enough for dermatitis herpetiformis, which requires additional prescription of specific drugs.
Thus, the relationship between the skin and intestinal processes in dermatitis herpetiformis remains unclear, although the parallelism of these disorders is obvious.
In recent decades, the infection Helicobacter pylori (H. pylori), initially associated with the development of chronic gastritis and peptic ulcers, has attracted particular attention. Subsequently, the possible role of this infectious agent in the development of a significant number of diseases, including skin diseases, was established (Table 3) [44].
A significant number of studies have been devoted to studying the relationship between H. pylori infection and chronic urticaria, which is a chronic skin disease characterized by recurrent itchy blisters without a clear connection with physical and nutritional factors. The nature of this relationship has not been fully established. One hypothesis suggests an increase in intestinal permeability against the background of Helicobacter pylori infection to various agents that can cause mast cell degranulation [45]. Other hypotheses attribute immunomodulatory effects to H. pylori. In particular, IgG and IgA antibodies to the H. pylori-associated 19-kDa lipoprotein were detected in the blood serum of patients with chronic urticaria [46, 47]. Autoantibodies of the IgG class to IgE and Fc-εRiα are also detected, and the possibility of production of antibodies during Helicobacter pylori infection due to molecular mimicry is assumed [48]. Studies evaluating the effectiveness of H. pylori eradication in chronic urticaria are controversial. However, a carefully designed study by E. Magen et al. showed a significant decrease in process activity in patients who received treatment compared with those who did not receive treatment [49].
In this regard, the importance of H. pylori in the development of atopic dermatitis is discussed. Thus, G. Corrado et al. showed a positive association of food allergy with Helicobacter pylori infection in a group that included 30 children with various gastrointestinal complaints [50]. In a study by IH Galadari et al. the frequency of detection of H. pylori in patients with atopic dermatitis was significantly higher compared to controls [51]. K. Murakami et al. described a case of successful treatment of atopic dermatitis in a 14-year-old girl who underwent H. pylori eradication [52]. However, many aspects of this relationship remain controversial.
It is also assumed that Helicobacter pylori infection may be a trigger for the development of psoriasis [54–56], but the mechanisms of its development are unknown.
Thus, diseases of the digestive system have a significant impact on the condition of the skin, and symptoms of its damage may indicate damage to the gastrointestinal tract. The mechanisms of this relationship may be different, and in some cases remain completely unknown. However, with the development of a skin pathological process, diseases of the digestive system should be excluded, and with gastroenterological pathology, the condition of the skin should be closely monitored, which may be important for assessing the activity of the process, its prognosis and prescribing adequate therapy in both gastroenterological and dermatological practice .
Literature
- Hiliary W. Observations on the Changes of the Air and the Concomitant Epidemic Diseases in the Island of Barbados. London: Hitch and Harris, 1759.
- Whitfield A. On a hitherto undescribed disease of the skin // Brit. J. Derm. 1932; 44:24–28.
- Wells GC Skin disorders in relation to malabsorption // Brit. med. J. 1962; 2:937–943.
- Thrash B., Patel M., Shah KR, Boland CR, Menter A. Cutaneous manifestations of gastrointestinal disease. Part II // J Am Acad Dermatol. 2013; 68: 211.e1–33.
- Naveh Y., Lightman A., Zinder, O. Effect of diarrhea on serum zinc concentrations in infants and children // J. Pediatr. 1982; 101:730–732.
- Black RE, Sazawal S. Zinc deficiency and zinc supplementation for childhood diarrhea in developing countries // J. Am. Coll. Nutr. 1998; 17:516.
- Wapnir RA Zinc Deficiency, Malnutrition and the Gastrointestinal Tract // J. Nutr. 2000; 130: 1388S-1392S.
- Ghishan FK Transport of electrolytes, water and glucose in zinc deficiency // J. Pediatr. Gastroenterol. Nutr. 1984; 3: 608–612.
- Lee RG, Rains TM, Tovar-Palacio C., Beverly JL, Shay NF Zinc deficiency increases hypothalamic neuropeptide Y and neuropeptide Y mRNA levels and does not block neuropeptide Y-induced feeding in rats // J. Nutr. 1998; 128:1218–1223.
- Humbert P., Pelletier F., Dreno B., Puzenat E., Aubin F. Gluten intolerance and skin diseases // Eur J Dermatol. 2006; 16:4–11.
- Abenavoli L., Proietti I., Leggio L., Ferrulli A., Vonghia L., Capizzi R., Rotoli M., Amerio PL, Gasbarrini G., Addolorato G. Cutaneous manifestations in celiac disease // World J Gastroenterol. 2006; 12:843–852.
- Lied GA Gastrointestinal food hypersensitivity: Symptoms, diagnosis and provocation tests // Turk J Gastroenterol. 2007; 18 (1): 5–13.
- Zar S., Kumar D., Benson MJ Review article: food hypersensitivity and irritable bowel syndrome // Aliment Pharmacol Ther. 2001; 15:439–449.
- Psachey RDG, Creamer B., Pierce JW Sclerodermatous involvement of the stomach and the small and large bowel // Gut. 1969; 10: 285–292.
- Marks J., Shuster S. Intestinal malabsorption and the skin // Gut. 1971, 12:938–947.
- Carron DB, Douglas AP Steatorrhoea in vascular insufficiency of the small intestine // Quart. J. Med. 1965; 34331–34340.
- Booth CC The enterocyte in coeliac disease // Brit. Med. J. 1970; 4:1–17.
- Marks J., Shuster S. Intestinal malabsorption and the skin // Gut. 1971; 12:938–947.
- Veloso FT, Carvalho J., Magro F. Immune-related systemic manifestations of inflammatory bowel disease: A prospective study of 792 patients // J Clin Gastroenterol. 1996; 23:29–34.
- Bernstein CN, Blanchard JF, Rawsthorne P., Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study // Am J Gastroenterol. 2001; 96:1116–1122.
- Rankin GB, Watts HD, Melnyk CS, Kelley ML, Jr. National Cooperative Crohn's disease study: extraintestinal manifestations and perianal complications // Gastroenterology. 1979; 77:914–920.
- Rothfuss KS, Stange EF, Herrlinger KR Extraintestinal manifestations and complications in inflammatory bowel diseases // World J Gastroenterol. 2006; 12:4819–4831.
- Veloso FT Review article: skin complications associated with inflammatory bowel disease // Aliment Pharmacol Ther. 2004; 20(Suppl 4): 50–53.
- Clayton TH, Walker BP, Stables GI Treatment of chronic erythema nodosum with infliximab // Clin Exp Dermatol. 2006; 31:818–825.
- Loftus EV Management of extraintestinal manifestations and other complications of inflammatory bowel disease // Curr Gastroenterol Rep. 2004; 6:506–513.
- Regueiro M., Valentine J., Plevy S., Fleisher MR, Lichtenstein GR Infliximab for the treatment of pyoderma gangrenosum associated with inflammatory bowel disease // Am J Gastroenterol. 2003; 98: 1821–1826.
- Mintz R., Feller ER, Bahr RL, Shah SA Ocular manifestations of inflammatory bowel disease // Inflamm Bowel Dis. 2004; 10: 135–139.
- Evans PE, Pardi DS Extraintestinal manifestations of inflammatory bowel disease: focus on the musculoskeletal, dermatologic, and ocular manifestations // MedGenMed. 2007. 19; 9 (1): 55–56.
- Jeghers H., McKusick VA, Katz KH Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance // N Engl J Med. 1949; 241(25):993.
- Lindor NM, Greene MH The concise handbook of family cancer syndromes. Mayo Family Cancer Program // J Natl Cancer Inst. 1998; 90 (14): 1039–171.
- Bishop P., Loftis S., Nowicki M. What syndrome is this? Peutz–Jeghers syndrome // Pediatr Dermatol. 2004; 21 (4): 503–505.
- Hemminki A., Markie D., Tomlinson I., avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Höglund P., Järvinen H., Kristo P., elin K ., Ridanpää M., Salovaara R., Toro T., Bodmer W., Olschwang S., Olsen AS, Stratton MR, De La Chapelle A., Aaltonen LA A serine/threonine kinase gene defective in Peutz–Jeghers syndrome // Nature. 1998; 391 (6663): 184–187.
- Spigelman AD, Thomson JP, Phillips RK Towards decreasing the relaparatomy rate in the Peutz–Jeghers syndrome: the role of preoperative small bowel endoscopy // Br J Surg. 1990; 77(3):301–302.
- Lin BC, Lien JM, Chen RJ, Fang JF, Wong YC Combined endoscopic and surgical treatment for the polyposis of Peutz–Jeghers syndrome // Surg Endosc. 2000; 14 (12): 1185–1187.
- Zbuk KM, Eng C. Hamartomatous polyposis syndromes // Nat Clin Pract Gastroenterol Hepatol. 2007; 4 (9): 492–502.
- Giardiello FM, Trimbath JD Peutz–Jeghers syndrome and management recommendations // Clin Gastroenterol Hepatol. 2006; 4 (4): 408–415.
- Erbe RW Inherited gastroinestinal-polyposis syndromes // N Engl J Med. 1976; 294(20):1101–1104.
- Utsunomya J., Gocho H., Miyanaga T., Hamaguchi E., Kashimure A. Peutz–Jeghers syndrome: its natural course and management // Johns Hopkins Med J. 1975; 136(2):71–82.
- Kaukinen K., Collin P., Maki M. Latent coeliac disease or coeliac disease beyond villous atrophy? // Gut. 2007; 56(10):1339–1340.
- Troncone R., Jabri B. Coeliac disease and gluten sensitivity // Journal of Internal Medicine. 2011; 269(6):582–590.
- Skripkin Yu. K., Sharapova G. Ya. Skin and venereal diseases. M.: Medicine, 1987, 168–177.
- Warren SJP, Cockerell CJ Characterization of a subgroup of patients with dermatitis herpetiformis with nonclassical histologic features // American Journal of Dermatopathology. 2002; 24 (4): 305–308.
- Smith AD, Streilein RD, Hall III RP Neutrophil CD11b, L-selectin and Fc IgA receptors in patients with dermatitis herpetiformis // British Journal of Dermatology. 2002; 147(6):1109–1117.
- Hernando-Harder AC, Booken N., Goerdt S., Singer MV, Harder H. Helicobacter pylori infection and dermatologic diseases // J Dermatol. 2009; 19 (5): 431–444.
- Buhner S., Reese I., Kuehl F., Lochs H., Zuberbier T. Pseudoallergic reactions in chronic urticaria are associated with altered gastroduodenal permeability // Allergy. 2004; 59: 1118–1123.
- Bakos N., Fekete B., Prohaszka Z., Fust G., Kalabay L. High prevalence of IgG and IgA antibodies to 19-Da Helicobacter pylori-associated lipoprotein in chronic urticaria // Allergy. 2003; 58:663–667.
- Mini R., Figura N., D'Ambrosio C. et al. Helicobacter pylori immunoproteomes in case reports of rosacea and chronic urticaria // Proteomics. 2005; 5:777–787.
- Appelmelk BJ, Simoons-Smit I, Negrini R et al. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity // Infect Immun. 1996; 64:2031–2040.
- Magen E., Mishal J., Schlesinger M., Scharf S. Eradication of Helicobacter pylori infection equally improves chronic urticaria with positive and negative autologous serum skin test // Helicobacter. 2007; 12:567–571.
- Corrado G., Luzzi I., Lucarelli S. et al. Positive association between Helicobacter pylori infection and food allergy in children // Scand J Gastroenterol. 1998; 33:1135–1139.
- Galadari IH, Sheriff MO The role of Helicobacter pylori in urticaria and atopic dermatitis // Skinmed. 2006; 5: 172–176.
- Murakami K., Fujioka T., Nishizono A. et al. Atopic dermatitis successfully treated by eradication of Helicobacter pylori // J Gastroenterol. 1996; 31(Suppl 9): 77–82.
- Valencak J., Trautinger F., Fiebiger WC, Raderer M. Complete remission of chronic plaque psoriasis and gastric marginal zone B-cell lymphoma of MALT type after treatment with 2-chlorodeoxyadenosine // Ann Hematol. 2002; 81:662–665.
- Saez-Rodriguez M., Noda-Cabrera A., Garcia-Bustinduy M. et al. Palmoplantar pustulosis associated with gastric Helicobacter pylori infection // Clin Exp Dermatol. 2002; 27:720.
- Raderer M., Osterreicher C., Machold K. et al. Impaired response of gastric MALT-lymphoma to Helicobacter pylori eradication in patients with autoimmune disease // Ann Oncol. 2001; 12:937–939.
N. G. Korotky, Doctor of Medical Sciences, Professor N. M. Narinskaya S. V. Belmer1, Doctor of Medical Sciences, Professor
GBOU VPO RNIMU im. N. I. Pirogova Ministry of Health of the Russian Federation, Moscow
1 Contact information
Abstract. Alimentary organ diseases have strong impact on skin condition and the symptoms of its affection may indicate affection of alimentary organ. The article covers relations between bowel pathology and changes of cutaneous covering in cases of Brandt's syndrome, Peutz–Jeghers' syndrome, Crohn's disease, interconnection of dermatitis herpetiformis and celiac disease. Authors also analyze the role of H. pylori in the development of atopic dermatitis.
About the causes of hormonal imbalance
In young men, the causes of hormonal disorders are associated with the following factors:
- The functioning of the thyroid gland, pituitary gland, testicles or other glands responsible for the production of hormones is disrupted. Failures can be caused by congenital pathologies or acquired anomalies.
- The gonads stopped producing hormones normally due to bruises, tumor processes, and exposure to infectious pathogens.
- Toxic effects of aggressive liquids, household chemicals and cosmetics.
- Antisocial lifestyle, alcohol or drug addiction, heavy smoking.
In older men, the main causes of androgen deficiency lie in the fact that the function of the gonads decreases, as a result of which less testosterone is produced. At the same time, the amount of estrogens (female hormones) increases and obesity develops. A vicious circle is formed when a decrease in androgens leads to obesity, which increases the synthesis of leptin (the hormone of adipose tissue), which further inhibits the synthesis of testosterone.
Note! Drinking large amounts of beer often leads to hormonal imbalances.
Regardless of age, disruption of hormone production may be associated with the following factors:
- poor physical activity;
- cardiovascular diseases;
- kidney pathologies;
- elevated cholesterol and glucose levels;
- poor nutrition;
- obesity;
- frequent stress, insomnia, chronic fatigue;
- overheating of the testicles (including with cryptorchidism).