Today, human papillomavirus (HPV) is one of the most common viral infections. According to statistics, it is present in almost 90% of the world's population. Scientists have also proven that two types of the virus (16 and 18) occur in approximately 70% of patients diagnosed with cervical cancer and precancerous pathological conditions of the cervix.1 It has been established that in women with long-term persistence of the virus in the body, the risk of developing cervical cancer increases 80 times. For this reason, doctors pay increased attention to the fight against this disease.
- Types of virus that can cause cancer
- Varieties of the virus
- Viferon suppositories for HPV Release form and composition
- Indications for use
- Suppository dosages
- Use during pregnancy
Human papillomavirus (HPV) belongs to the genus Papillomavirus and belongs to subgroup A of the Papovaviridae family. It inhabits the deep layers of the epithelium (skin and mucous membranes), and its reproduction occurs in the upper layers of the epithelium. The entire life cycle of the virus takes place only inside the cells of the body, but for some time it can also exist in the external environment, which explains its high contagiousness - the ability of infectious diseases to be transmitted from sick people to healthy people by transmitting the pathogen through direct contact or through transmission factors. Re-infection is possible because immunity to this virus is not developed.
When can the medicine be used?
The drug is indicated for the treatment of respiratory infections of viral etiology. The medicine effectively relieves symptoms of influenza, respiratory syncytial, rhinovirus and other infections. It is actively used to treat COVID-19, including in children, pregnant and lactating women. The medication can be used even with the addition of bacterial flora:
- Inflammation of the membranes and tissue of the brain;
- Infection in the womb (cytomegalovirus, herpes, candidiasis, chlamydia, etc.);
- Pneumonia;
- Pathologies of the reproductive organs (trichomoniasis, ureaplasmosis, HPV and others).
Viferon can be prescribed together with antibacterial and antifungal medications, as it increases the immune defense of the entire body.
The product is effective against viral intestinal diseases, namely: rotavirus, enterovirus. When using the drug, vomiting, nausea, abdominal pain, diarrhea, and intoxication quickly go away.
The medicine is often used for herpetic rashes caused by Herpes simplex types 1 and 2. The product relieves itching and rash.
The drug can be used even in newborns and premature infants. It does not cause serious complications and is non-toxic.
Viferon in the treatment and prevention of acute respiratory viral infections
AND
interferons (IFNs) were discovered in 1957 by English scientists A. Isaacs and S. Lindenmann as proteins that determine the phenomenon of interference, which consists in the body’s immunity to re-infection with the virus.
Over the half-century history of the development of the doctrine of the interferon system, many important issues of its functioning have been revealed, and nonspecific and specific mechanisms of resistance have been studied in detail. Experimental work has shown that the INF system, which arose in vertebrates during the process of phylogenesis, is involved in ensuring the constancy of the internal environment of the body - homeostasis [1]. INFs are active proteins that are produced by all cells of the body, but 99% of all INFs are produced by blood and bone marrow cells.
Interferons are formed under the influence of the antigenic system, but a small amount of them is produced without the participation of antigens. It is known, for example, that the level of INF is increased in patients with rheumatoid arthritis and systemic lupus erythematosus [2, 3].
The medical significance of INF is determined by their antiviral and immunocorrective activity, as well as antitumorigenic and radioprotective effects.
The interaction of IFN with cells begins with the binding of interferon to specific interferon receptors on the surface of cells. It has been established that different cells are sensitive to different types of interferons [4]. IFN-a and -b have a common receptor on the cell surface, while IFN-g has a separate receptor. Once inside the cell, IFN activates genes encoding effector proteins that are responsible for the antiviral effect. The role of IFN in antiviral protection is confirmed by the presence of a strict correlation between the level of production of endogenous IFN and recovery, aggravation of the severity of the disease when the activity of endogenous interferon is suppressed, and, finally, treatment with interferon helps prevent the development of viral infection [5].
IFNs are divided into three main types: a, b and g. a- and b-IFNs have an antiviral effect, inhibiting the processes of transcription and translation, disrupting the synthesis of viral proteins [6].
Evidence has been obtained of a connection between the interferon system and the immune system: interferons are important mediators of immunity
, which allows them to be classified as a family of regulatory cytokines, stimulates phagocytosis, natural killer cell activity, and enhances the expression of histocompatibility antigens of classes 1 and 2 [7].
The antiviral and immunomodulatory activity of IFN was the basis for the use of interferon drugs in medicine. The most effective for most infectious diseases are interferons-a, which are used very widely in clinical practice. First of all, this applies to second-generation INF - recombinant interferons-a2, obtained by genetic engineering (without the use of donor blood). There are several interferon-a2 preparations: 2a, 2b and 2c.
Interferon preparations are quite effective, but the consequence of the biological processes that IFNs cause in the body are adverse reactions. The most common (with large individual variations) is influenza-like syndrome, observed in almost all patients, especially in cases of high doses of INF. Along with flu-like syndrome, long-term courses may cause serious side effects from the cardiovascular system (hypotension, heart rhythm disturbances) and the central nervous system (irritability, aggressiveness or depression). All this limits the use of IFN drugs, and in case of dangerous side effects requires discontinuation.
The search for new forms of drugs to avoid negative phenomena led to the creation of Viferon
– a new antiviral immunomodulatory drug developed at the Research Institute of Epidemiology named after. N.F. Gamaleya RAMS (produced by Feron LLC, Moscow). In Russia, Viferon was registered and approved for use in 1998. The dosage form is rectal suppositories and ointment.
Mechanism of action of Viferon
Viferon is a complex of recombinant interferon-a2b in combination with antioxidant drugs - a-tocopherol acetate and ascorbic acid in therapeutically effective doses.
In a series of experimental works by V.V. Malinovskaya showed that the ability to produce IFN increases significantly with the addition (in vitro) of α-tocopherol acetate and ascorbic acid, which influence the processes of lipid peroxidation of cell membranes, to IFN-inducing cells [7]. The use of the drug combination effectiveness index formula showed that the use of R-IFNa with antioxidants had a synergistic effect on the antiviral effect of R-IFNa, greatly enhancing the antiviral effect of interferon [8].
A detailed study of the mechanism of action of Viferon revealed its immunomodulatory effect on T- and B-lymphocytes, normalization of humoral immunity, and the content of immunoglobulin E [9]. A clear correlation has been established between various factors of specific, nonspecific and antioxidant protection in the treatment of viral and bacterial infections [10].
A study of the pharmacokinetics of the drug showed that rectal administration of Viferon made it possible to achieve higher concentrations and prolonged circulation of R-IFN in the blood than with parenteral administration [11]. A decrease in the level of serum interferon after 12 hours necessitates its repeated administration.
Side effects and tolerability
Side effects of Viferon were not recorded during the drug trial.
It has been established that long-term use of Viferon does not produce antibodies that neutralize the antiviral activity of R-IFN alpha 2b.
Currently, Viferon is the only drug from the class of recombinant interferons registered in Russia, approved by the State Pharmacological Committee of the Ministry of Health of the Russian Federation for the treatment of various infectious diseases not only in adults, but also in children, including newborns, as well as pregnant women.
Clinical and immunological effectiveness of Viferon
Virus-induced immune dysfunction (T-cell suppression, inhibition of phagocytosis, etc.), which develops with influenza and other acute respiratory viral infections, allows us to classify the latter as one of those diseases, the course and outcome of which are determined by the presence of immune drugs in complex treatment.
In our clinic, over the past years, the clinical effectiveness of Viferon in respiratory diseases and their bacterial complications has been studied. In particular, in an open, placebo-controlled study, we demonstrated high therapeutic efficacy for influenza and other respiratory diseases complicated by sore throat [12]. 54 patients with lacunar tonsillitis and acute respiratory viral disease, confirmed by laboratory methods, were under observation. Reliable evidence has been obtained of the effectiveness of treatment with antibacterial agents in combination with Viferon. The drug was prescribed at a dose of 500 thousand IU twice a day for five days. Treatment was started no later than 48 hours from the appearance of the first signs of the disease. The therapeutic effect was expressed in a reduction in the symptoms of intoxication and the duration of purulent deposits on the tonsils, and a reduction in the duration of the disease in general.
The clinical effect, as a rule, was accompanied by a pronounced improvement in immunological parameters: normalization of initially reduced DM3 and DM4, and a decrease in the number of circulating immune complexes (Table 1). The positive dynamics of circulating immune complexes during treatment with Viferon, from our point of view, is an important criterion indicating the elimination of the pathogen and a reduction in the duration of the disease on the one hand [12].
According to other clinical observations of 137 patients aged 1 to 14 years with severe forms of ARVI, based on clinical follow-up analysis, the authors found that in severe forms of infection, a low initial level of serum IFN and a significant decrease in the total number of T- lymphocytes, decreased activity of phagocytes, which is the basis for prescribing Viferon from 1–2 days of illness to children with severe forms of ARVI.
Follow-up observation of 50 children examined for 3–6 months revealed a protective effect of Viferon against subsequent episodes of ARVI [13]. The authors recommend prescribing the drug to frequently and long-term ill children aged 1–14 years for 5 days, 2 suppositories per day in the following doses: 500,000 IU per suppository for children under 7 years of age, and 1 million IU for children from 7 to 14 years of age , and use it for preventive purposes during periods of high incidence of ARVI and for the adaptation of children in preschool institutions.
Clinical and immunological observations in a group of adults and children who often suffer from viral and bacterial infections of the respiratory tract made it possible to identify disturbances in the interferon system: a decrease in the ability of leukocytes to produce a- and g-interferons. Pronounced changes in the T-cell immune system require the use of adequate doses of Viferon [13,15]. The frequency and duration of course treatment depend on the severity of the disease and the severity of changes in the immune status. As a rule, therapy with Viferon is carried out over several months at an age-specific dosage. Prolonged viferon therapy for 2.5 months made it possible to reduce the incidence of ARVD by approximately 2–5 times per year [13].
Use of Viferon ointment for the treatment and prevention of influenza and other acute respiratory viral infections
Viferon ointment, containing 40 thousand IU of IFN and tocopherol acetate in a therapeutic dose per 1 g, is used at the first signs of the disease intranasally 3-4 times a day for 5 days. For the purpose of prevention in areas of infection, the ointment is applied in a thin layer to the mucous membrane of the nasal passages 2 times a day, morning and evening, throughout the entire dangerous period.
According to the results of the study by E.S. Makarova (2001), conducted in a preschool institution, when studying the interferon status in children from 1 year 7 months to 3 years against the background of the preventive use of Viferon ointment, an increase in the production of g-interferon, a significant decrease in the amount of circulating “early” interferon, and a reduction in morbidity by 4 times, no adverse reactions. The results obtained, according to the authors’ conclusion, indicate the high effectiveness of viferon prophylaxis in frequently ill young children [15].
Thus, convenient dosage forms, high therapeutic and prophylactic effectiveness of Viferon, and the focus of its effect on the immune system make it possible to recommend this drug in the complex treatment and prevention of respiratory infections.
In 2001, work on the creation of new dosage forms of recombinant interferons (including Viferon) and their introduction into clinical practice was awarded a prize from the Government of the Russian Federation.
Among herbal remedies for the prevention of ARVI, preparations such as dry purified extract from the leaves of sea buckthorn of the sucker family are recommended. The drug has antiviral and interferon-inducing activity and is prescribed to adults and children at the first symptoms of a respiratory disease. The most pronounced therapeutic effect is achieved with a combination of dosage forms of general resorptive and local action.
Echinacea purpurea juice, which contains a unique complex of phytocomponents, is effective.
The drug stimulates the body’s production of its own interferon, prevents the development of the disease and alleviates its symptoms; Available in the form of drops, it is recommended to take a course of at least a week. Achieving a lasting effect – after 8 weeks. Literature:
1. Soloviev V.D. Bektemirov T.A. Interferons in the theory and practice of medicine. 2nd ed. – M.1981.
2. Cross JC, Roberts RM Constitutive and trophoblast – specific expression if a class of bovine interferon genes. Proc. Natl. Acad. Sci USA 1991: 88, 3817 – 3824.
3. Dianzani F. The interferon system. Health Sciences Press, 1993
4. Khesin Ya.E., Narovlyansky A.N., Amchenkova A.M. Cellular receptors for interferons. In the book. The interferon system is normal and in pathology. Moscow, 1996: 39 – 52.
5. Dianzani F. Baron S. The interferon's: a biological system with therapeutic potential in viral infections. In Lymphoblastoid Alpha – Interferon Current
Medical Literature Ltd, London, 1995.
6. Ershov F.I. Interferons (to the 40th anniversary of the discovery). Question virus., 1998, No. 6, vol. 43, p. 247 – 252.
7. Himmler A., Hauptmann R. et al. Structure and expression in Escherichia coli of canine interferon alpha gene. J. Interferon Res, 1987: 7, p 173 – 178.
8. Malinovskaya VV “Interferon a2b antivirus action modulation with the help of antioxidant action preparation” International Conference on Interferon's Biology and Clinical Applications. 77, 1998.
9. Tareeva T. G. et al. “The use of Viferon for intrauterine infection of the fetus and newborns” Abstract. Dokl. Russian national congress “Man and Medicine” M., 162, 1995.
10. Keshinyan E.S. and others. “The use of genetically engineered alpha 2-interferon - Viferon in the complex therapy of severe forms of infectious and inflammatory diseases in newborns” Abstract.
report Russian national congress "Man and Medicine", 162, 1995.
11. Babayants A.A. Malinovskaya V.V. Meshkova E.N. “Pharmacokinetics of interferon after rectal administration” Issue. Virus.1:83–84, 1986
12.. Kolobukhina L.V. Gatich R.Z. Merkulova L.N. and others. “Complex treatment of acute respiratory viral infections complicated by tonsillitis” Lech. Doctor, 2003, No. 1, pp. 32–33.
13. Chebotareva T.A. Timina V.P. Malinovskaya V.V. Viferon: use for influenza and other ARVI in children in the book. “Immunomodulatory and antiviral drug Viferon in the treatment of children and adults who often suffer from viral-bacterial infections,” M. 2003.
14. Karaulov A.V. Sokurenko S.I. Barmotin G.V. The principle of immunotherapy and immunorehabilitation of recurrent respiratory diseases. Medical doctor, 2000, No. 1, pp. 44 – 45.
15. Makarova E.S. Doskin V.A. Malinovskaya V.V. and others. The use of Viferon in young children depending on the seasonal rhythms of interferon formation. Abstracts of the report. Ross. scientific Cong. “Man and Medicine” (M), April 2 – 6, 2001, p. 272.
Instructions for use
The medicine in suppositories is used rectally (the suppository is inserted into the anus). To insert suppositories correctly, you need to spread your buttocks and insert a suppository into the anus. After administering the medicine, you should lie down for half an hour. During this time the candle will dissolve.
Now we will tell you how to place a Viferon suppository rectally for a baby. For children, suppositories are administered in a position on their side (newborns) or on their stomach (children over 1 year). To administer the medication, spread the buttocks and insert a suppository into the anus. Next, squeeze the gluteal muscles and hold the child in this position for 2-3 minutes (since the candle may slip out). After administering the drug, the baby should lie down for half an hour.
Clinical pharmacology
Biological properties: Human recombinant interferon alpha-2 has pronounced antiviral, immunomodulatory and antiproliferative properties. The complex composition of the drugs (ointment, suppository, gel) causes a number of new additional effects: in the presence of antioxidants (tocopherol acetate and/or ascorbic acid, benzoic or citric acids, as well as methionine), the specific antiviral activity of human recombinant alpha-2 interferon increases, its immunomodulatory effect on T- and B-lymphocytes, the level of immunoglobulin E is normalized, and the functioning of the endogenous interferon system is restored. Alpha-tocopherol acetate, ascorbic acid, benzoic and citric acids, as well as methionine, being highly active antioxidants, have pronounced anti-inflammatory, membrane-stabilizing and regenerating properties. It has been established that when using the drug Viferon rectal suppositories, there are no side effects that occur with parenteral administration of interferon preparations, and antibodies that neutralize the antiviral activity of interferon are not formed.
Can Viferon be used by children?
Viferon suppositories can be used by children of different ages. Doses of medication for infection in newborns and premature infants:
- Prematures with a gestational age of more than 34 weeks, 150,000 IU twice a day. The interval between doses is 12 hours. The duration of course therapy is 5 days.
- Premature babies less than 34 weeks - 150,000 IU three times a day (every 8 hours) for 5 days.
Children under 7 years of age with ARVI are also advised to take Viferon 150,000 IU twice a day. Course therapy lasts up to 5 days. If treatment is ineffective, you should see a pediatrician.
The number of courses of treatment may vary depending on the type of disease:
- flu - 1-2,
- cytomegalovirus infection - 2-3,
- enterovirus infection - 2-3,
- pneumonia (as a complication after a viral infection) - 1-2,
- herpes - 2.
The number of courses is determined by the doctor. The interval between them is 5-6 days.
According to research, with coronavirus, children under 7 years of age can be prescribed up to 1,000,000 IU 2 times a day. And for children over 8 years old, up to 3,000,000 IU twice a day. The duration of therapy is 7-14 days, depending on the severity of the pathology. Treatment with high doses allows you to quickly remove the pathogen from the body.
For viral hepatitis B, C, D, it is recommended to prescribe Viferon suppositories:
- Under 6 months of age: 3,000,000-5,000,000 IU per day;
- Up to 12 months of age: 5,000,000 IU per day;
- 1-7 years: 3,000,000 per 1 square meter of body surface area per day;
- Over 7 years of age - 5,000,000 IU per 1 square meter of body surface area per day.
In the first 1.5 weeks of therapy, the medicine is taken twice a day. Next, candles are placed every other day for six months to a year.
Nosological classification (ICD-10)
- A41.9 Septicemia, unspecified - A49.3 Infection caused by mycoplasma, unspecified - A59.0 Urogenital trichomoniasis - A74.9 Chlamydial infection, unspecified - B00.0 Herpetic eczema - B00.1 Herpetic vesicular dermatitis - B18 Chronic viral hepatitis - B25. 9 Cytomegalovirus disease, unspecified - B34 Viral infection of unspecified localization - B37.3 Candidiasis of the vulva and vagina (N77.1) - G03.9 Meningitis, unspecified - J06.9 Acute upper respiratory tract infection, unspecified - J11 Influenza, virus not identified - J18 Pneumonia, unspecified clarification of the pathogen - N39.0 Urinary tract infection without established localization - N76 Other inflammatory diseases of the vagina and vulva - O35.3 Damage to the fetus (suspected) as a result of a viral disease of the mother, requiring medical care for the mother - O35.8 Other anomalies and lesions of the fetus ( suspected) requiring provision of medical care to the mother
Treatment of various infections in adult patients
In adults, for ARVI, influenza and other pathologies of viral etiology, 500,000 IU is prescribed twice a day. Therapy lasts 5-10 days. If treatment needs to be continued, after completing the first course, take a break for 5 days, after which the therapy is repeated.
Pathologies caused by the herpes virus are indicated to be treated with a dosage of 1,000,000 IU twice a day. The course of treatment lasts 1.5 weeks. With frequent relapses, the course of therapy can last more than 1.5 weeks.
During pregnancy with a viral urogenital infection (including herpes), it is recommended to put a suppository of 500,000 IU twice a day for 1.5 weeks. Then twice a week, 1,000,000 IU per day (course therapy - 1.5 weeks). After a month, preventive therapy is indicated at a dose of 150,000 IU twice a day for 5 days. It is possible to repeat prophylaxis before childbirth.
For viral hepatitis, the medicine is indicated in a dose of 3,000,000 IU twice a day every day for 1.5 weeks. Afterwards, a maintenance course of 3,000,000 IU is prescribed every other day for six months to a year. The duration of therapy is determined by the doctor depending on the results of laboratory tests and the patient’s well-being.
For the drug to work correctly, you need to know where to store Viferon suppositories. The medication should be stored in a cool place so that the candle keeps its shape and does not melt. The optimal temperature is 2–8 °C.
Contraindications and side effects
The ointment and gel have no contraindications, with the exception of individual intolerance. They should not be used by patients who may experience rashes, redness or other types of allergies. If such consequences occur, therapy should be stopped immediately.
It is necessary to consult a doctor for further advice. Symptomatic treatment is not indicated, since all symptoms disappear within 2-3 days. The standard course duration is 7 days. After this, you can take a break and continue therapy only in consultation with your doctor.
The drug can be used in any form, starting from the 14th week of pregnancy. During breastfeeding, Viferon can be used without restrictions. The ointment and gel are safe for health if the instructions are followed. Cases of overdose have not been registered to date.
Ointment and gel - rules of use
Ointment or gel is often used to treat herpes on the skin and mucous membranes. A single dosage is 0.5 cm. The drug is applied to the skin or mucous membrane (nasal passages, tonsils) 3-5 times a day. The surfaces to be treated must be pre-cleaned. The duration of therapy is 5 days. External agents are indicated simultaneously with Viferon suppositories.
The gel can be used to treat inflammatory processes of the cervix. The product is applied twice a day. Before use, it is recommended to clean the cervical mucosa with a cotton swab so that there is no mucus. The course of therapy is 1-2 weeks. The gel is also used as part of the general treatment of cervicitis.
Attention! The tube should be stored in a cool place. Otherwise, the effectiveness of treatment is reduced.
For preventive purposes, external forms are recommended to be applied to the tonsils or nasal mucosa twice a day. Course therapy lasts up to 1 month.
Gel Viferon 36,000 ME
Ointment "Viferon": composition, description
This is an antiviral drug that strengthens the immune system. The composition contains interferon protein, which belongs to the alpha-2b type. Available in different forms:
- ointment;
- candles;
- gel.
It is used for the prevention and treatment of diseases associated with viruses. Thanks to the destruction of pathogenic microorganisms, patients can use significantly less hormonal drugs and antibiotics, which makes them feel better and has a positive effect on their health.
The effect on the body is complex:
- antiviral – for the treatment of herpes, influenza, hepatitis, among others;
- restoration of the interferon synthesis system;
- antibacterial – treatment of mixed infections;
- stimulation of systems that synthesize antibodies;
- strengthening the immune system.
The drug stabilizes the membranes of body cells, promotes tissue regeneration, and has anti-inflammatory and powerful antioxidant effects. This contributes not only to cure from infectious pathologies, but to relatively rapid recovery.
The ointment or gel can only be stored in the refrigerator at a temperature in the range of 2-8°C. The place should be moderately damp and dark. Access for children is prohibited. The drug can be used within the expiration date, which is 2 years from the date of production.
special instructions
The drug should not be used for generalized or atypical herpetic infections. Since the medicine can cause an allergic reaction, it is not prescribed for atopic dermatitis or eczematous rashes. The drug is not recommended for:
- tumor processes on the skin,
- familial pemphigus Gougereau-Hailey,
- mental disorders,
- simultaneous use of sedative drugs,
- neutropenia less than 1.5×109,
- the platelet count in the blood is less than 90,000/µl,
- diseases in which there is a disruption of the central nervous system.
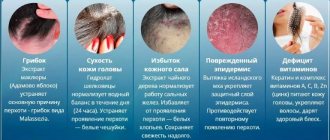
Symptoms of anal warts
A burning sensation in the anus is a symptom of the appearance of anal warts.
Although many people infected with HPV are asymptomatic, doctors have long known the full list of “classic” manifestations of this disease. In addition to anal warts, carriers of the papilloma virus often voice the following complaints:
- itching and burning in the anus;
- hyperemia;
- cracks and suppuration in the anal area;
- discharge from the anus;
- uncharacteristic pain that appears during anal sex.
In the presence of concomitant diseases (for example, HIV), HPV symptoms can increase many times over.
Some side signs of the virus may also begin to appear. For example, wart growths, when infected, begin to emit an unpleasant odor and provoke the formation of fistulas in the anus, starting the process of intoxication of the entire body.
This, in turn, leads to discharge from the anus of ichor mixed with pus and the appearance of sharp, almost unbearable pain during defecation.
Analogues of the drug Viferon
Viferon has its own structural and non-structural analogues. Structural analogues include Grippferon, Kipferon. They also contain human recombinant interferon alpha-2b. Grippferon differs only in the release form (drops, spray). Kipferon is produced in candles.
Is it possible to combine Viferon and Kipferon? The combined use of drugs is not recommended, since they have the same active ingredient. With simultaneous use, an overdose or an allergic reaction is possible.
Non-structural analogues include:
- Anaferon - contains antibodies to human interferon gamma; less effective, allowed from 1 month;
- Arbidol - indicated from 3 years of age, for example, for coronavirus and influenza, has a less pronounced clinical effect in relation to acute respiratory viral infections, and is not used at all for bacterial infections, since it has a targeted effect on viral particles;
- Kagocel is an interferon inducer, affects the production of its own interferons, and is not indicated for infants and children under 3 years of age.
Viferon is the most effective medicine among all antiviral drugs. It has proven itself in the treatment of influenza, coronavirus and other acute respiratory viral infections in adults and children. Due to the lack of toxicity and undesirable effects, it can be used in pregnant women and newborns. The medicine is ideal for the prevention of viral diseases and is used as part of the general treatment of bacterial infections.
Composition and release form
Ointment for external and local use 1 g
- recombinant human interferon alpha-2 40000 IU - tocopherol acetate 0.002 g - excipients: anhydrous lanolin;
medical Vaseline; peach oil; purified water in aluminum tubes of 12 g; 1 tube in a cardboard box. Gel for local use 1 ml
- recombinant human interferon alpha-2 36000 IU - excipients: alpha-tocopherol acetate solution - 5%; methionine alcohol solution - 2%; benzoic acid solution - 0.4%; citric acid - solution - 10%; sodium tetraborate solution - 3%; sodium chloride solution - 10%; human serum albumin solution - 10%; glycerin - distilled; sodium carboxymethylcellulose; purified water - in aluminum tubes of 10 ml; 1 tube in a cardboard pack
Suppositories for rectal use 1 sup.
- human recombinant interferon alpha-2: - 150,000 IU - 500,000 IU - 1,000,000 IU - 3,000,000 IU - excipients: ascorbic acid - 0.015 g (150,000 IU), 0.022 g (500,000 IU, 1,000,000 IU, 3,000,000 IU ); tocopherol acetate - 0.055 g; base - cocoa butter or solid fat in PVC/PVC strip packaging of 10 pcs.; in a cardboard pack 1 package.