Dalatsin gel for external use 1% 30 g
Pharmacological group:
Antibiotic lincosamide ATC code: D10AF01.
Pharmacodynamics:
Mechanism of action: Clindamycin is an antibiotic of the lincosamide group that inhibits the synthesis of bacterial proteins. It binds to the 50S ribosomal subunit and influences ribosome assembly and translation. Although clindamycin phosphate is inactive in vitro, in vivo it is rapidly hydrolyzed to form clindamycin, which has antibacterial activity. Clindamycin has in vitro activity against isolates of the following microorganisms. Anaerobic gram-positive, non-spore-forming, rod-shaped bacteria, including: Propionibacterium acnes.
Pharmacodynamic effects: Efficacy depends on the duration of the period during which the level of the active substance exceeds the minimum inhibitory concentration (MIC) for the pathogen (% T/MIC).
Resistance: Resistance to clindamycin in Propionibacterium acnes may be caused by mutations in the rRNA binding site of the antibiotics or by methylation of specific nucleotides in the 23S RNA of the 50S ribosomal subunits. These changes may explain cross-resistance to macrolides and streptogramin B (MLSB phenotype). Macrolide-resistant isolates should be tested for inducible resistance to clindamycin using the D test. Cross-resistance of microorganisms to clindamycin and lincomycin has been demonstrated. The prevalence of acquired resistance may vary geographically and over time, and it is desirable to have information on regional patterns of selected microbial species, particularly when treating severe infections. If necessary, expert opinion should be obtained if the spread of resistant species in the region calls into question the applicability of the drug for the treatment of at least some types of infections. In particular, in cases of severe infections or treatment failure, microbiological diagnosis is recommended to confirm the pathogen and its sensitivity to clindamycin. Resistance is usually determined by interpretation of susceptibility criteria (breakpoints) established by regulatory authorities, CLSI or EUCAST for systemically administered antibiotics. These breakpoints may be less relevant for topical clindamycin. Although clindamycin was not specifically mentioned, the European Committee on Antibacterial Susceptibility Testing (EUCAST) has suggested that for topical antibiotics, resistance is better determined by epidemiological cut-off values (ECOFF) than by epidemiological cut-off values (ECOFF). clinical breakpoints intended for systemic use. However, MIC distributions and ECOFF values have not been published by the EUCAST committee for P. acnes. Based on the correlation between clinical findings in acne patients and clindamycin MICs of their P. acnes isolates, values up to 256 mg/L are considered sensitive to systemic clindamycin. CLSI has published MIC ranges for a limited number (58) of unique clinical isolates of P. acnes obtained in 2010–2012. in US hospitals; 91% of these isolates were susceptible to clindamycin (MIC > 8 mg/L). A recent Belgian observational study (2011–2012) of anaerobic bacteria included 22 P. acnes isolates; 95.5% were sensitive to clindamycin. An earlier European observational study that included 304 P. acnes isolates reported an incidence of clindamycin resistance of 15%. However, this study used a cutoff value of 0.12 mg/L; if the current breakpoint of 4 mg/L is used, there will be no resistant isolates.
Pharmacokinetics:
After topical application of 1% clindamycin phosphate gel, very low concentrations of clindamycin are detected in the blood serum and urine. Clindamycin has been shown to be active in comedones in patients with acne vulgaris. The average antibiotic concentration in the comedonal contents after applying a solution of clindamycin in isopropyl alcohol and water (10 mg/ml) for 4 weeks averaged 597 μg/g of comedonal contents (0-1490 μg/g). The sensitivity of all tested strains of Propionibacterium acnes to clindamycin in vitro was shown (MIC 0.4 μg/ml). After clindamycin is applied to the skin, the amount of free fatty acids on the skin surface decreases from approximately 14% to 2%.
Use in Elderly Patients Clinical studies did not include a sufficient number of patients over 65 years of age to be able to assess whether there are differences in pharmacokinetics in elderly patients compared to younger patients.
Dalacin vaginal cream 2% 20 g tube 1 pc. In Volgograd
Pharmacological action: Binds to the 50S ribosomal subunit of the microbial cell and inhibits protein synthesis of sensitive microorganisms. It has a bacteriostatic effect; in high concentrations and against highly sensitive microorganisms it can exhibit a bactericidal effect. According to the mechanism of action and antimicrobial spectrum, it is close to lincomycin (2–10 times more active against some types of microorganisms).
When taken orally, clindamycin hydrochloride is quickly and well absorbed from the gastrointestinal tract (better than lincomycin), bioavailability is 90%, simultaneous food intake slows down absorption without changing the degree of absorption. Protein binding - 92–94%. Easily penetrates biological fluids, organs and tissues of the body, incl. tonsils, muscle and bone tissue (approximately 40% of the concentration in the blood), bronchi, lungs, pleura, pleural fluid (50–90%), bile ducts, appendix, fallopian tubes, prostate gland, synovial fluid (50%), saliva , sputum (30–75%), wound discharge. It passes through the BBB poorly (with inflammation of the meninges, the permeability of the BBB increases). The volume of distribution in adults is approximately 0.66 l/kg, in children - 0.86 l/kg. Quickly passes through the placenta, is found in the fetal blood (40%), penetrates into breast milk (50–100%).
Clindamycin palmitate and clindamycin phosphate are inactive; they are quickly hydrolyzed in the body to active clindamycin.
Cmax in blood serum after oral administration is achieved after 0.75–1 hour, after intramuscular administration - after 3 hours (adults) or 1 hour (children), with intravenous infusion - by the end of administration. Metabolized in the liver to form active (N-dimethylclindamycin and clindamycin sulfoxide) and inactive metabolites. It is excreted within 4 days in the urine (10%) and through the intestines (3.6%) in the form of the active fraction, the rest - in the form of inactive metabolites. T1/2 with normal renal function in adults is 2.4-3 hours, in infants and older children - 2.5-3 hours, in premature newborns - 6.3-8.6 hours. In end-stage renal disease failure or in case of severe liver dysfunction, the elimination of clindamycin is slowed down (T1/2 in adults - 3-5 hours). Does not accumulate.
When 100 mg of clindamycin phosphate was administered intravaginally as a 2% vaginal cream once daily for 7 days in 5 women with bacterial vaginosis, systemic absorption was approximately 5% (range 2–8%) of the administered dose. Cmax values on the first day are approximately 13 ng/ml (from 3 to 34 ng/ml), on the seventh day - on average 16 ng/ml (from 7 to 26 ng/ml); Tmax - approximately 16 hours (range 8–24 hours) after application. With repeated intravaginal use, systemic accumulation was absent or insignificant. T1/2 for systemic absorption - 1.5–2.6 hours.
When clindamycin phosphate is administered intravaginally as a suppository at a dose of 100 mg once daily for 3 days, approximately 30% (6–70%) of the administered dose is absorbed into the systemic circulation, with an average AUC of 3.2 mcg/h/ml (0.42–11 mcg/h/ml). Cmax is reached approximately 5 hours (1–10 hours) after insertion of the vaginal suppository.
When used as a topical gel, clindamycin phosphate is rapidly hydrolyzed by phosphatases in the ducts of the sebaceous glands to form clindamycin. The gel can be absorbed in quantities causing systemic effects.
Sensitive to clindamycin in vitro
the following microorganisms: aerobic gram-positive cocci, including
Staphylococcus aureus, Staphylococcus epidermidis,
incl.
strains that produce and do not produce penicillinase ( in vitro
, rapid development of resistance to clindamycin has been noted in some staphylococcal strains resistant to erythromycin),
Streptococcus spp.
(except
Streptococcus faecalis
);
Pneumococcus spp.;
anaerobic gram-negative bacilli, including
Bacteroides spp
.
(including the B. fragilis
and
the B. melaninogenicus
),
Fusobacterium spp.;
anaerobic gram-positive bacilli that do not form spores, including
Propionibacterium spp., Eubacterium spp., Actinomyces spp.;
anaerobic and microaerophilic gram-positive cocci, including
Peptococcus spp., Peptostreptococcus spp., Microaerophilic Streptococcus spp., Clostridia spp.
(Clostridia are more resistant to clindamycin than most other anaerobes).
Most Clostridium perfringens
are sensitive to clindamycin, but other species, such as
C. sporogenes
and
C. tertium,
are often resistant to clindamycin, so susceptibility testing is necessary.
In high doses it affects some protozoa (Plasmodium falciparum)
.
Cross-resistance has been shown between clindamycin and lincomycin and antagonism between clindamycin and erythromycin.
In vitro
clindamycin is active against most strains of the following microorganisms that cause bacterial vaginosis:
Gardnerella vaginalis, Mobiluncus spp., Mycoplasma hominis, Bacteroides spp., Peptostreptococcus spp.
Intravaginal clindamycin is not effective for the treatment of vulvovaginitis caused by
Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, Candida albicans,
or Herpes simplex virus
.
The anti-acne effect when applied topically is probably due to the fact that clindamycin reduces the concentration of free fatty acids on the skin and inhibits the proliferation of Propionibacterium acnes
- an anaerobic found in the sebaceous glands and follicles.
P. acnes
strains to clindamycin
in vitro
(MIC 0.4 μg/ml).
Carcinogenicity, mutagenicity, effect on fertility
Long-term animal studies have not been conducted to evaluate the potential carcinogenicity of clindamycin. No mutagenic effect was detected in the Ames test and micronucleus test on rats. No adverse effects on fertility or mating ability were observed in rats receiving oral clindamycin at doses up to 300 mg/kg/day (approximately 1.6 times the MRDI in mg/m2 terms).
Pregnancy.
In a reproduction study on animals (rats, mice) using oral doses of clindamycin up to 600 mg/kg/day (3.2 and 1.6 times higher than the MRDC in terms of mg/m2, respectively) or subcutaneous doses up to 250 mg/kg/day (1.3 and 0.7 times higher than the MRDC in terms of mg/m2, respectively) no teratogenic effect was detected. In one experiment on mice, cleft palates were noted in fetuses (this result was not confirmed in experiments on other animals and on other strains of mice).
Dalatsin gel for external use 1% tube 30g
Compound
Active ingredient: clindamycin phosphate 11.88 mg (in the form of clindamycin 10 mg);
Excipients: allantoin 2 mg, methyl parahydroxybenzoate 3 mg, propylene glycol 50 mg, macragol 100 mg, carbomer 934P 7.5 mg, sodium hydroxide 40%, purified water qs up to 1 g.
Pharmacokinetics
After topical application of 1% clindamycin phosphate gel, very low concentrations of clindamycin are detected in serum and urine.
Clindamycin has been shown to be active in comedones in patients with acne vulgaris. The average antibiotic concentration in the comedonal contents after applying a solution of clindamycin in isopropyl alcohol and water (10 mg/ml) for 4 weeks averaged 597 μg/g of comedonal contents (0-1490 μg/g). The sensitivity of all studied strains of Propionibacterium acnes
to clindamycin
in vitro
(MIC 0.4 μg/ml). After clindamycin is applied to the skin, the amount of free fatty acids on the skin surface decreases from approximately 14% to 2%.
Use in elderly patients
Clinical studies did not include a sufficient number of patients over 65 years of age to be able to assess whether there are differences in pharmacokinetics in older patients compared to younger patients.
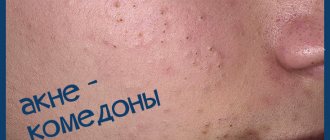
Indications for use
Acne vulgaris.
Contraindications
- History of hypersensitivity to clindamycin or lincomycin.
- In patients with a history of antibiotic-associated colitis.
- Age up to 12 years (data on safety and effectiveness are not available).
Directions for use and doses
Externally. A thin layer of gel is applied to the affected area of clean, dry skin 2 times a day.
Course of treatment: to obtain satisfactory results, treatment should be continued for 6-8 weeks, and if necessary, can be continued for up to 6 months. If after using the drug for several months the effectiveness of treatment decreases, then you should take a break of 4 weeks.
Storage conditions
At a temperature not exceeding 25o C, out of the reach of children. Do not freeze.
Best before date
2 years. Do not use after the expiration date indicated on the package!
special instructions
Avoid getting the drug on the mucous membrane of the eyes and in the oral cavity. After applying the gel, wash your hands thoroughly. In case of accidental contact with sensitive surfaces (eyes, skin abrasions, mucous membranes), rinse the area thoroughly with cool water.
The use of clindamycin (as well as other antibiotics) orally or parenterally is in some cases associated with the development of severe diarrhea and pseudomembranous colitis. With external use of clindamycin, cases of diarrhea and colitis are rare, however, caution should be exercised, and if severe or prolonged diarrhea develops, the drug should be discontinued and, if necessary, appropriate diagnostic and therapeutic measures should be taken. Typically, the onset of diarrhea, colitis, and pseudomembranous colitis occurs within a few weeks of completion of oral or parenteral clindamycin therapy. In case of severe diarrhea, the question of whether a colonoscopy is advisable should be decided. The use of drugs that reduce gastrointestinal motility, such as opioid analgesics and diphenoxylate with atropine, may prolong and/or worsen the course of this complication. Vancomycin has been found to be effective against antibiotic-associated pseudomembranous colitis caused by Clostridium difficile .
The usual dose, divided into 3 to 4 doses for adults, is 500 mg to 2 g of vancomycin per day orally for 7 to 10 days.
Description
Antibiotic - lincosamide.
Pharmacodynamics
Mechanism of action Clindamycin is an antibiotic of the lincosamide group that inhibits the synthesis of bacterial proteins. It binds to the 50S ribosomal subunit and influences ribosome assembly and translation. Although clindamycin phosphate is inactive in vitro, in vivo
it is rapidly hydrolyzed to form clindamycin, which has antibacterial activity.
Clindamycin has in vitro
against isolates of the following microorganisms.
Anaerobic gram-positive, non-spore-forming, rod-shaped bacteria, including: Propionibacterium acnes.
Pharmacodynamic effects Efficacy depends on the duration of the period during which the level of the active substance exceeds the minimum inhibitory concentration (MIC) for the pathogen (%T/MIC).
Resistance Resistance to clindamycin in Propionibacterium acnes
can be caused by mutations in the site where antibiotics bind to rRNA or by methylation of specific nucleotides in the 23S RNA of the 50S ribosomal subunits.
These changes may explain cross-resistance to macrolides and streptogramin B (MLSv phenotype). Macrolide-resistant isolates should be tested for inducible resistance to clindamycin using the D test. Cross-resistance of microorganisms to clindamycin and lincomycin has been demonstrated. The prevalence of acquired resistance may vary geographically and over time, and it is desirable to have information on regional patterns of selected microbial species, particularly when treating severe infections. If necessary, expert opinion should be obtained if the spread of resistant species in the region calls into question the applicability of the drug for the treatment of at least some types of infections. In particular, in cases of severe infections or treatment failure, microbiological diagnosis is recommended to confirm the pathogen and its sensitivity to clindamycin. Resistance is usually determined by interpretation of susceptibility criteria (breakpoints) established by regulatory authorities, CLSI or EUCAST for systemically administered antibiotics. These breakpoints may be less relevant for topical clindamycin. Although clindamycin was not specifically mentioned, the European Committee on Antibacterial Susceptibility Testing (EUCAST) has suggested that for topical antibiotics, resistance is better determined by epidemiological cut-off values (ECOFF) than by epidemiological cut-off values (ECOFF). clinical breakpoints intended for systemic use. However, MIC distributions and ECOFF values have not been published by the EUCAST committee for P. acnes. Based on the correlation between clinical results in acne patients and clindamycin MICs of their P. acnes
, values up to 256 mg/L are considered sensitive to systemic clindamycin.
CLSI has published MIC ranges for a limited number (58) of unique clinical isolates of P. acnes
obtained in 2010–2012. in US hospitals; 91% of these isolates were susceptible to clindamycin (MIC > 8 mg/L). A recent Belgian observational study (2011–2012) of anaerobic bacteria included 22 P. acnes isolates, 95.5% were sensitive to clindamycin. An earlier European observational study that included 304 P. acnes isolates reported an incidence of clindamycin resistance of 15%. However, this study used a cutoff value of 0.12 mg/L; if the current breakpoint of 4 mg/L is used, there will be no resistant isolates. BreakpointsThe CLSI and EUCAST breakpoints for gram-positive anaerobic microorganisms are listed below. Although the two institutes present different values, the resistance breakpoint is the same, as CLSI has recognized an intermediate susceptibility category (4 mg/L). As stated above, these breakpoints are based on use in systemic infections. EUCAST breakpoints for clindamycin for systemic use
CLSI breakpoints for systemic clindamycin
Side effects
* Adverse reactions recorded during post-registration use of the drug.
If any of the side effects indicated in the instructions are aggravated or any other side effects not listed in the instructions are noted, you should immediately inform your doctor.
Use during pregnancy and breastfeeding
In animal studies, when clindamycin was administered subcutaneously or orally, there was no impairment of fertility, as well as any negative effects on the fetus, except when the drug was taken in doses toxic to the mother. Results from animal studies cannot always be extrapolated to humans. In clinical studies, with systemic administration of the drug to women during the second and third trimesters of pregnancy, no increase in the incidence of congenital anomalies of the fetus was noted. No studies have been conducted on the use of the drug in women during the first trimester of pregnancy. The drug should be used during pregnancy only if the expected benefit to the mother outweighs the potential risk to the fetus.
It is not known whether clindamycin is excreted into breast milk after topical use. Clindamycin has been found in breast milk at concentrations <0.5 - 3.8 mcg/ml after systemic use.
Clindamycin has the potential to cause adverse effects on the gastrointestinal flora of breastfed infants, such as diarrhea or blood in the stool, or rash. If a nursing mother requires the use of clindamycin, this is not a reason to stop breastfeeding, but an alternative drug may be preferred. The developmental and health benefits of breastfeeding should be considered, as well as the clinical need for clindamycin in the mother and any potential adverse effects related to clindamycin or the underlying maternal condition in the breastfed infant.
Interaction
There is cross-resistance of microorganisms to clindamycin and lincomycin.
Antagonism between clindamycin and erythromycin has been noted.
It has been established that clindamycin, when used systemically, disrupts neuromuscular transmission and, therefore, can enhance the effect of other peripherally acting muscle relaxants, so the drug should be used with caution in patients receiving drugs of this group.
Overdose
When applied topically, clindamycin can be absorbed in amounts causing systemic effects. Possible systemic side effects include diarrhea, hemorrhagic diarrhea, including pseudomembranous colitis.
In case of overdose, symptomatic and supportive therapy is indicated.
Impact on the ability to drive vehicles and operate machinery
There is no reason to believe that the use of the drug Dalacin may affect the ability to drive a car or operate machinery.