Release form, packaging and composition of the drug Dysport®
| Lyophilisate for the preparation of solution for injection | 1 fl. |
| botulinum toxin type A complex - hemagglutinin | 500 units* |
Excipients: human albumin - 125 mcg, lactose monohydrate - 2.5 mg.
Glass bottles (1) in a cardboard holder - cardboard packs.
* ED is a unit of activity of the company.
Clinical and pharmacological group:
Muscle relaxant. Acetylcholine release inhibitor
Pharmacotherapeutic group:
MIBP
Indications for the drug Dysport®
- blepharospasm, hemifacial spasm, spasmodic torticollis, spasticity of the arm muscles after a stroke, hyperkinetic folds (expression wrinkles) of the face in adults;
- dynamic foot deformity caused by spasticity in cerebral palsy in children aged 2 years and older;
- hyperhidrosis of the axillary region.
| ICD-10 code | Indication |
| G24.3 | Spasmodic torticollis |
| G24.5 | Blepharospasm |
| G80 | Cerebral paralysis |
| R25.2 | Cramp and spasm |
| R61 | Hyperhidrosis |
Dosage regimen
Bilateral and unilateral blepharospasm, hemifacial spasm
The contents of a bottle of 300 units are diluted in 1.5 ml of 0.9% sodium chloride solution for injection, the contents of a bottle of 500 units are diluted in 2.5 ml of 0.9% sodium chloride solution for injection. 1 ml of both solutions contains 200 units of the drug Dysport®.
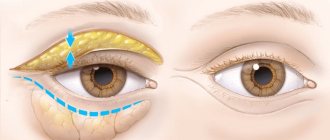
For adults and elderly patients for the treatment of bilateral blepharospasm, the recommended starting dose is 120 units per eye. The drug is administered subcutaneously in a volume of 0.1 ml (20 units) medially, in a volume of 0.2 ml (40 units) laterally into the connection between the preseptal and orbital parts of the upper and lower parts of the circular muscle (m.orbicularis oculi) of the affected eye. For injections into the upper eyelid, the needle should be directed away from the center so as not to touch the muscle that lifts the upper eyelid (m.levator palpebrae superioris). Below is a diagram showing where the injections will take place.
The clinical effect can be expected within 2-4 days, the maximum therapeutic effect develops within 2 weeks.
Injections should be repeated every 12 weeks or as indicated to prevent recurrence of symptoms. With each subsequent administration, the dose of the drug should be reduced to 80 units per eye. For example, 0.1 ml (20 units) medially and 0.1 ml (20 units) laterally above the eye and below the eye. In the future, the dose of the drug can be reduced to 60 units per eye, by eliminating the introduction of the drug medially into the lower eyelid. The doctor determines subsequent doses in accordance with the effect obtained.
For unilateral blepharospasm, injections should be limited to the area of the affected eye. Similar treatment is carried out for hemifacial spasm.
Spasmodic torticollis
The contents of a bottle of 300 units are diluted in 0.6 ml of 0.9% sodium chloride solution for injection, and a bottle of 500 units is diluted in 1 ml of 0.9% sodium chloride solution for injection. 1 ml of both solutions contains 500 IU of the drug Dysport®.
Doses recommended for the treatment of torticollis are used in adults of all ages who have normal body weight and satisfactory neck muscle development. A reduction in the dose of the drug is possible in case of significant underweight or in elderly people with reduced muscle mass.
For the treatment of spastic torticollis, the initial total single dose is 500 units. This dose is distributed between the 2 or 3 most active muscles of the neck.
For rotational torticollis, the drug in a dose of 500 units is administered as follows: 350 units into the splenius capitis muscle, ipsilateral to the direction of head rotation, and 150 units into the sternocleidomastoid muscle, contralateral to the rotation.
For laterocollis (head-to-shoulder tilt), a dose of 500 IU is distributed as follows: 350 IU is injected ipsilaterally into the splenius capitis muscle (m. splenius capitis) and 150 IU - ipsilaterally into the sternocleidomastoid muscle (m. sternocleidomastoideus). In cases involving elevation of the shoulder by the trapezius muscle (m.trapezius) or the muscle that lifts the scapulae (m.levator scapulae), treatment may be required according to visible muscle hypertrophy or according to electromyography.
When injection of the drug into 3 muscles is required, a dose of 500 IU is distributed as follows: 300 IU is injected into the splenius capitis muscle, 100 IU into the sternocleidomastoid muscle (m.sternocleidomastoideus), 100 IU into the third muscle (trapezius or levator scapula).
For anterocollis (head tilt forward), 150 units are injected into both sternocleidomastoid muscles (m. sternocleidomastoideus).
For retrocollis (tilting the head back), a dose of 500 units is distributed as follows: 250 units are injected into each splenius capitis muscle. In case of insufficient clinical effect after injection, after 6 weeks the drug can be injected into the trapezius muscles (m.trapezius) bilaterally (at a dose of up to 250 IU per muscle). Bilateral injections into the splenius capitis muscle may increase the risk of developing neck muscle weakness.
When subsequently prescribing the drug, doses can be adapted in accordance with the clinical effect and side effects encountered. Recommended total doses are 250-1000 units. Use of the drug in higher doses may be accompanied by an increase in the frequency of side effects, in particular dysphagia.
Clinical improvement in spastic torticollis is observed within 1 week after injection. Injections should be repeated every 8-12 weeks or as needed.
For the treatment of other forms of torticollis, the use of electromyography (EMG) is of great importance to identify and administer the drug to the most active muscles. EMG should be used to diagnose all complex forms of torticollis or when re-examining patients with no positive dynamics after administration of the drug, for injections into deep muscles and in patients with excess body weight and difficult to palpate neck muscles.
Arm spasticity after stroke in adults
The indications for administration of the drug Dysport® in the treatment of arm spasticity after a stroke are determined by a neurologist 3 months after the stroke.
0.6 ml of 0.9% sodium chloride solution is injected into a bottle with the drug containing 300 units, and 1 ml of 0.9% sodium chloride solution is injected into the bottle with the drug containing 500 units. In both cases, receiving a solution containing 500 units of the drug Dysport® in 1 ml.
The maximum total single dose is 1000 IU, which is distributed between the following 5 muscles: flexor digitorum profundus, flexor digitorum superficialis, flexor carpi ulnaris, flexor radialis wrist (m.flexor carpi radialis) and biceps brachii (m.biceps brachii).
When choosing an injection site, one should be guided by standard EMG points, and the immediate injection site is determined by palpation. In all muscles, except the biceps brachii muscle, injections are made at one point. The injection is carried out into the biceps brachii muscle at 2 points. The recommended dose distribution between muscles is shown in the table.
| Muscles | Dysport® (IU) |
| Biceps brachii | 300-400 |
| Flexor digitorum profundus | 150 |
| Flexor digitorum superficialis | 150-250 |
| Flexor carpi ulnaris | 150 |
| Flexor carpi radialis | 150 |
| Total dose | 1000 |
The initial total dose of the drug can be reduced to 500 units to prevent excessive weakness of the injected muscles in cases where the target muscles are small in volume, when an injection into the biceps brachii muscle is not performed, or when patients are given an injection over several points of one muscle.
Clinical improvement occurs within 2 weeks after injection. Injections can be repeated approximately every 16 weeks or as needed to maintain effect, but no more frequently than every 12 weeks.
Hyperkinetic folds (expression wrinkles) of the face
The main area of application of Dysport® for cosmetic correction is the upper half of the face. The lower half of the face and neck are corrected by injecting botulinum toxin much less frequently.
The contents of the 300 IU bottle are diluted with 1.5 ml of 0.9% sodium chloride solution for injection, and the contents of the 500 IU bottle are diluted with 2.5 ml of 0.9% sodium chloride solution for injection. At this dilution, 1 ml of both solutions contains 200 units of the drug Dysport®.
The total recommended dose for a single administration in all four areas (the glabellar region, the forehead, the outer corner of the eye and the back of the nose) should not exceed 200 units of Dysport®.
To correct vertical folds in the eyebrow area, injections of the drug are made into the corrugator supercilii muscle, 8-10 units per 2-4 points, and into the procerus muscle, 5-10 units per 2 points. The total dose ranges from 42 to 100 units.
Elimination of hyperkinetic folds in the forehead area is carried out by injecting the drug into the area of maximum tension of the frontalis muscle (m.frontalis). The number of injection points can be arbitrary. All of them should be located 2 cm above the eyebrow line on the same line or in a V-shape. The optimal total dose of Dysport® in this area is 30-40 units (maximum - 90 units) at a rate of 5-15 units per point, the total number of points is 4-6.
Correction of folds in the area of the outer corner of the eye (“crow’s feet”) is carried out by subcutaneous injection into points located 1 cm lateral from the outer corner of the eye, at the rate of 5-15 IU of Dysport® per injection point. The number of points is from 2 to 4 for each eye. The maximum recommended total dose on both sides is 120 units.
The frequency of repeated injections depends on the timing of restoration of facial muscle activity. The duration of the effect is 3-4 months.
If an adequate dose of the drug was administered during the first injection, then during the second and subsequent injections the total dose of Dysport® can be reduced by 15-20 IU for the appropriate areas. In this case, it is possible to increase the interval between injections of the drug to 6-9 months. If the initial dose of the drug was insufficient, then with repeated injections it should be increased.
To correct wrinkles in the dorsum of the nose, injections are made into the middle of the belly of the nasal muscles. The dose is distributed at 5-10 units to 1-2 points in each muscle.
The muscle relaxant effect of the drug Dysport® on the facial muscles of the face is clinically manifested on days 2-3 after administration and reaches a maximum on days 14-15.
Recommended doses of Dysport® used in aesthetic medicine do not cause systemic side effects.
Dynamic foot deformity caused by spasticity in cerebral palsy in children aged 2 years and older
The contents of a bottle of 300 units are dissolved in 0.6 ml of 0.9% sodium chloride solution for injection, and the contents of a bottle of 500 units in 1 ml of 0.9% sodium chloride solution for injection, in both cases obtaining a solution containing 500 units in 1 ml.
The drug is administered intramuscularly into the calf muscles (m. gastrocnemius). The initial recommended dose is 20 units/kg body weight and is divided equally between the calf muscles (m. gastrocnemius). If one calf muscle is affected (m.gastrocnemius), the drug is administered at a dose of 10 U/kg. The optimal dose is determined individually; subsequent treatment should be planned after assessing the results of the initial dose. To avoid side effects, do not exceed the maximum dose of 1000 units. The drug is predominantly administered into the gastrocnemius muscle (m.gastrocnemius), but it is possible to inject it into the soleus muscle (m.soleus) and the tibialis posterior muscle (m.tibialis posterior). To determine the most active muscles, you can use the electromyography method.
In cases where the patient's target muscles are small in volume, the initial dose of the drug should be reduced to prevent the development of excessive weakness. Clinical improvement occurs within 2 weeks after administration of the drug. Injections are repeated as needed at intervals of at least 12 weeks, and the administered dose can vary from 10 to 30 units per 1 kg of body weight, depending on the effect of the previous injection.
Treatment of axillary hyperhidrosis
The contents of the 300 IU bottle are diluted with 1.5 ml of 0.9% sodium chloride solution for injection, and the contents of the 500 IU bottle are diluted with 2.5 ml of 0.9% sodium chloride solution for injection, obtaining in both cases a solution containing 200 IU in 1 ml.
The recommended starting dose is 100 units per axillary area. If the desired effect is not achieved, then a subsequent dose increase to 200 units is possible.
The area of drug administration is determined by Minor's test. The test is carried out before treatment and, if necessary, dynamically, at room temperature (22-24°C) after the patient has rested for 15 minutes. To carry out the test you need: 5% alcohol solution of iodine; potato starch; marker; antiseptic; brush; gauze napkins.
The patient is in a supine position with his hands under his head. The sweating area is treated with a 5% alcohol solution of iodine and after 1 minute a thin layer of potato starch is applied to this area with a napkin or brush. The test results are assessed after 5 minutes. In the presence of sweating, the treated surface is visually observed to turn blue. The intensity of color (from pale blue to blue-black) correlates with sweating activity. After the test, the area of hyperhidrosis is marked with a marker, then the starch is washed off with alcohol or another antiseptic.
Intradermal injections are carried out at ten points in each axillary area, 10 units of the drug in a volume of 0.05 ml are injected into each point, 100 units per area. The maximum therapeutic effect develops within 2 weeks. In most cases, the recommended starting dose suppresses sweating for up to 48 weeks. The frequency of repeated injections is determined individually when the initial level of sweating is restored, but not more often than once every 12 weeks. If there is any evidence of a cumulative effect with repeated injections, the timing of repeat injections is determined individually for each patient.
Factors affecting the removal of Botox
Cosmetologists do not know how much the active substance in Botox is removed from the body. Reason: characteristics of each patient. Depends on factors.
| Factor | Description |
| The immune system | Botox is poison for the human body. Result: activation of the enhanced functioning of the immune system, neutralization of harmful substances. Information: good immunity maintains the duration of action of Botox for 4 months. Weak indicator: prohibition of injection |
| Patient age category | Age category 50+ requires 1 Botox injection per year. Less - depends on the nature of the visit to the cosmetologist |
| Gender | In women, the active component is excreted longer. Reason: minimal activity |
| Metabolic rate | If high, the active substance is excreted quickly, otherwise – slowly |
| Problem area | The effect quickly wears off in the eye area. Other areas: 4-6 months |
Additionally: volume of the injected component. Result: the minimum dosage is eliminated within 3 months.
Rules for preparing solution for injection
Remove the protective plastic tamper evident cap from the bottle.
When diluting the drug, do not open the bottle by removing the stopper. Immediately before diluting the contents of the bottle, the central part of the stopper should be treated with alcohol. The lyophilisate is diluted by introducing a regulated volume of 0.9% sodium chloride solution for injection into the bottle by piercing the stopper with a sterile needle of size 23 or 25. The resulting solution is a colorless transparent liquid. Since the drug does not contain a preservative, it is recommended to use it immediately after dissolution. The diluted drug can be stored for no more than 8 hours at a temperature of 2° to 8°C.
Rules for processing tools and waste disposal
Immediately after the injection, the remaining solution in the vial or syringe should be inactivated with a dilute sodium hypochlorite solution containing 1% active chlorine. All ancillary materials that have come into contact with the drug should be disposed of in accordance with standard hospital practice.
Spilled medication should be removed with an absorbent cloth soaked in 1% sodium hypochlorite solution.
Positive effects of Dysport, how long will it last?
The first results can be seen in a few days. You will suddenly look more youthful, your skin will be smoother, and you will be more comfortable looking at yourself in the mirror. The effect of the injection will last about five to seven months. After the effect
It started to get worse from the injections, the manipulations need to be repeated. Dysport does not cause addiction and muscle atrophy is excluded. If you want to return your muscles to their former activity, return their condition to the previous level, then you just need to wait a little, the effect will disappear after a while on its own.
However, even if you stop taking the drug, some of the wrinkles will still go away, the skin will be renewed, and the oval of the face will become clearer and more youthful. What is this connected with? This happens because while your muscles are under the influence of the drug, you lose the habit of frowning and lose excessive emotionality. Therefore, the condition of your skin will be good, even if you do not want to carry out manipulations again. In addition, the general condition of the body will improve, since headaches and migraines can occur due to excessive expression of emotions.
Side effect
During various clinical studies of the drug Dysport® involving about 7800 patients, adverse reactions developed with the following frequency: very often (≥1/10), often (≥1/100, up to General side effects
From the nervous system: rarely - neuralgic amyotrophy.
Dermatological reactions: rarely - skin rash.
Local reactions: often - pain and hematoma at the injection site; uncommon - irritation, burning sensation at the injection site, which lasts 1-2 minutes.
General reactions: often - general weakness, fatigue, flu-like syndrome.
Arm spasticity in adults after stroke
Adverse reactions were reported in 14 clinical studies involving 141 patients.
From the digestive system: often - dysphagia (has been reported when doses exceeding 2700 units were used, administered at one point or distributed between several points of administration).
From the musculoskeletal system: often - weakness of the arm muscles.
Other: often - accidental injury/fall.
Dynamic foot deformity caused by spasticity in children with cerebral palsy
Adverse reactions were reported in 14 clinical studies involving approximately 900 patients.
From the digestive system: often - diarrhea.
From the musculoskeletal system: often - weakness of the leg muscles.
From the urinary system: often - urinary incontinence.
Other: often - accidental injury due to a fall and abnormal gait, which are a consequence of excessive muscle weakness and/or spread of the action of the toxin to other muscles close to the injection site involved or involved in a certain motor act and in maintaining the balance of the patient's body in a standing position and when walking.
Spasmodic torticollis
Adverse reactions were reported in 21 clinical studies involving approximately 4,100 patients.
From the nervous system: often - dysphonia; infrequently - headache.
From the side of the organ of vision: infrequently - diplopia, impaired accommodation.
From the respiratory system: rarely - respiratory disorders.
From the digestive system: very often - dysphagia; Uncommon: dry mouth.
Dysphagia has a dose-dependent effect and occurs most often when the drug is administered into the sternocleidomastoid muscle. A diet excluding roughage may be required until symptoms resolve
Blepharospasm and hemifacial spasm
Adverse reactions were reported in 13 clinical studies involving approximately 1,400 patients.
From the nervous system: often - weakness of the facial muscles; infrequently - paresis of facial muscles.
From the organs of vision: very often - ptosis; often - diplopia, dry eyes, lacrimation; rarely - ophthalmoplegia.
Dermatological reactions: often - swelling of the eyelids; rarely - turn of the century.
A side effect may occur if the doctor fails to comply with the rules for performing the injection (dilution, accurate calculation of the administered dose, correct choice of injection points, direction of the needle and depth of injection) and the associated excessive diffusion of the drug and temporary paralysis of the muscle groups adjacent to the injection site.
Axillary hyperhidrosis
Adverse reactions were reported in 4 clinical studies involving approximately 217 patients.
Dermatological reactions: often - compensatory sweating.
Hyperkinetic folds (expression wrinkles) of the face
The following adverse reactions have been reported (usually mild to moderate in intensity).
From the organ of vision: often - swelling of the eyelids and mucous membrane of the eye; infrequently - dryness of the mucous membrane of the eye (keratoconjunctivitis sicca).
From the musculoskeletal system: often - weakness of the muscles adjacent to the injection site, which also often leads to ptosis of the eyelids, asthenopia (weakness of vision) or, rarely, to paresis of the facial muscles and visual disturbances.
From the nervous system: very often - headache (also often observed in the placebo group).
Dermatological reactions: infrequently - rash, itching; rarely - urticaria.
Local reactions: very often - pain, hematoma, itching, paresthesia, erythema, rash at the injection site (which were also often observed in the placebo group).
Post-registration experience of use
Most side effects are mild and transient.
Rarely: digestive disorders, allergic skin reactions, dizziness, headaches.
Very rare (1/10,000): severe muscle weakness, dysphagia, aspiration pneumonia, which can be fatal.
Price
The price of the service is determined individually. There are many reasons.
| Factor | Peculiarity |
| Complication level | The abundance of side effects requires an integrated approach |
| Volume of active substance introduced into the patient’s body | The method of detoxification of the patient is determined |
| Methodology | Depends on the level and nature of complications |
| Medical Center | The clinic determines the cost independently |
| Rehabilitation period | The need is determined by the chosen technique |
| Region | Place of residence dictates the market price of services |
Help: Consultation with a specialist is recommended. Tip: registration is made through the administrator of the medical center. Advantages of a private clinic
Advantages: • individual approach; • reverent attitude; • professional doctors; • recording for any time; • low price; • guarantee of results; • professional equipment; • goodwill, focus on results. Additionally: no side effects, quick withdrawal of Botox.
Contraindications for use
- acute diseases (the drug is administered after recovery);
- pregnancy;
- lactation period (breastfeeding);
- hypersensitivity to the components of the drug.
Use during pregnancy and breastfeeding
Dysport® is contraindicated for use during pregnancy and lactation (breastfeeding).
Studies of the effect of Dysport® on reproduction and teratogenicity have not been conducted. The safety of using Dysport® during pregnancy and breastfeeding has not been confirmed.
Use in elderly patients
It is possible to reduce the dose of the drug in elderly people with reduced muscle mass.
Recommendations from Beauty Lounge Specialists
Before performing injection procedures, consultation with a specialist is required. A qualified cosmetologist will familiarize yourself with the skin condition, initial data and indicate the recommended treatment plan, including the drug and the number of units.
After the procedure, you should follow all the recommendations of specialists: for 7-10 days you should not massage or rub your face, overheat the treated area, sunbathe, swim in a pool or natural reservoirs, sleep with your face in a pillow, do other cosmetic procedures, and also take alcohol and spicy food .
special instructions
Treatment with Dysport® should be carried out by specialists who have experience in diagnosing and treating these diseases and who have been trained to administer treatment with this drug.
Particular caution should be taken when re-introducing the drug to patients who have had allergic reactions to the previous injection.
Side effects resulting from the action of the toxin on muscles distant from the injection site have been reported. Patients receiving Dysport® at therapeutic doses may experience general muscle weakness. The risk of such side effects can be reduced by following drug dosage recommendations and using the drug in the minimum effective doses.
The drug is prescribed with caution and under strict medical supervision to patients with subclinical or clinical manifestations of lesions of neuromuscular transmission (for example, bulbospinal palsy). Such patients may have an increased sensitivity to botulinum toxin preparations, which can cause severe muscle weakness in them.
Dysport® should be administered with caution to patients with impaired swallowing and breathing functions, because these disorders may be aggravated due to the widespread effect of the toxin on the corresponding muscles.
In rare cases, patients suffering from chronic respiratory diseases may develop aspiration.
Isolated cases of death caused by dysphagia (impaired swallowing), pneumopathy, or in patients with significant asthenia have been reported during therapy with botulinum toxins type A and B.
Patients and those caring for them should be warned about the need to urgently consult a doctor in cases of swallowing, speech and breathing problems.
The formation of anti-botulinum antibodies was observed in a small number of patients who were treated with Dysport®. Clinically, this was manifested by a decrease in the therapeutic effect, which required a constant increase in drug doses.
In patients with a slow blood clotting time and inflammation at the intended injection site, Dysport® should be used in cases of extreme necessity.
The units of action of Dysport® are specific and cannot be compared with those of other drugs containing botulinum toxin.
Impact on the ability to drive vehicles and operate machinery
There are no data on the effect of the drug on the ability to drive vehicles and operate machinery.
Can I withdraw it quickly?
Reason: lack of result. Kinds:
- regular massages and treatments. Principle: gentle massage, high intensity. Result: impact on epidermal cells, restoration of the muscle layer. Recommendations: lymphatic drainage;
- physiotherapy. The principle is based on stimulation of damaged muscles with minimal current pulses. Help: Darsonval and myostimulation are prescribed. Effect: rapid increase in muscle performance, improved blood circulation in the subcutaneous tissue. Consequences: rapid withdrawal of the active component;
- physical exercise.
- Additionally: aerobics class. Action: regular performance of gymnastic exercises – facelifting. Result: impact on the drug injection sites. Tip: regularly turn your head to the sides.