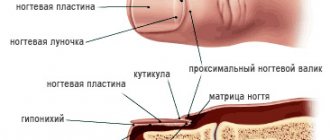
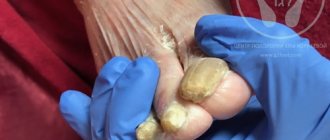
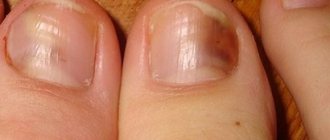
- Onychomycosis (fungal nail infection).
- Mycoses of the scalp.
- Fungal skin infections - treatment of dermatomycosis of the trunk, legs, feet, as well as yeast infections of the skin caused by the genus Candida (for example, Candida albicans) - in cases where the location, severity or extent of the infection determine the advisability of oral therapy.
A new broad-spectrum systemic antifungal drug is Lamisil (terbinafine), which is an allylamine compound; Available in 250 mg tablets.
Manufactured by the Swiss company Novartis. Among systemic antimycotics, only Lamisil has a fungicidal effect in therapeutic concentrations against dermatophytes, molds and some dimorphic fungi.
The effect on yeast can be fungicidal or fungistatic depending on the type of fungus. The fungicidal effect of Lamisil is achieved by inhibiting the enzyme squalene epoxidase in the cell membrane at the early stage of sterol biosynthesis in the fungal cell. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. The drug does not affect the metabolism of hormones or other drugs.
99% of Lamisil is bound to plasma proteins, its inactive metabolites are excreted in the urine.
Lamisil has the highest activity against dermatophytes among systemic antimycotics
,
which are most often the causative agents of fungal diseases of the skin and nails - in 85-94% of cases.
The minimum
fungicidal
concentration of Lamisil against dermatophytes is 0.004 mcg/ml (Rukavishnikova V.M., Sukolin G.I., Kuklin V.T.), the minimum
inhibitory
concentration (MIC) against dermatophytes: Epidermophyton floccosum - 0.001-0.006 mcg/ ml; Microsporum spp. - 0.002-0.01 µg/ml, Trichophyton spp. - 0.001-0.01 µg/ml (NS Ryder). The effectiveness of Lamisil in the treatment of onychomycosis caused by yeast is 60-84%.
Due to its high lipophilicity, Lamisil accumulates in the stratum corneum of the dermis, hair follicles, hair, and skin rich in sebaceous glands in concentrations that provide a fungicidal effect. The drug penetrates into the nail through the matrix and nail bed.
In nail plates, Lamisil remains in concentrations much higher than the MIC for another 30 weeks after 6-week therapy for onychomycosis and 36-48 weeks after 12-week therapy (F. Schatz et al.).
The frequency of mycological and clinical cure of superficial mycoses with Lamisil, according to domestic and foreign scientists, is 80-96%.
Lamisil is more effective than griseofulvin, itraconazole and fluconazole in terms of clinical and mycological cure of patients with onychomycosis.
In April 1999, data from a study were published that compared the effectiveness and safety of continuous therapy for onychomycosis with Lamisil with the effectiveness and safety of pulse therapy with itraconazole. The study involved 38 research centers in 6 European countries. The study was headed by the President of the European Association of Dermatologists and Venereologists, Professor G. Evans. This study is abbreviated LION ( L
amisil vs.
I
traconasole in
On
ychomycoses).
The study included about 500 patients and was carried out over a period of one and a half years. To date, this study has been published in one of the most popular European journals - the British Medical Journal and is recognized by the world dermatological community as the most authoritative study of the comparative effectiveness of systemic antimycotics.
Based on this study, the following conclusions were drawn.
- Lamisil provided a higher percentage of cure for onychomycosis compared to itraconazole.
- Cure in the group of patients receiving Lamisil therapy occurred faster.
- Patients and doctors rated the results of treatment with Lamisil more highly than with itraconazole.
Conclusion: In summary, continuous terbinafine therapy provides 3 significant benefits in the treatment of onychomycosis. Considering all the benefits together, terbinafine stands out strongly compared to itraconazole pulse therapy:
- Terbinafine has been confirmed to be more effective with a low relapse rate.
- Good tolerance.
- Higher profitability.
Lamisil in the treatment of onychomycosis
ABOUT
Nichomycosis is one of the most common human diseases. It is currently believed that nail diseases caused by fungi occur in 50% of patients with lesions of the nail plates. In Russia, the number of patients with onychomycosis varies from 4.5 to 15 million people.
The causative agents of onychomycosis are dermatophytes, yeasts, and molds; however, their relationship in the spectrum of morbidity is assessed by researchers differently. In the vast majority (91.3%) of patients, dermatophytes are identified – primarily Tr. rubrum
(72%), less often –
Tr.
menthagrophytes (19.3%) [1, 15, 21] (Fig. 1).
In particular, Tr.
rubrum , as a rule, is the cause of damage to the nail plates of the feet;
yeasts usually affect fingernails, and non-dermatophytic molds, which cause 3–5% of onychomycosis, affect the feet [23]. Mostly yeasts and molds are found in combination with Tr.
rubrum .
Rice. 1. Proportions of pathogens in the etiology of onychomycosis
Predisposing factors for the development of mycotic infection include vascular diseases of the lower extremities (obliterating endarteritis, thrombophlebitis, varicose veins, Raynaud's syndrome), systematic microtrauma of the nail plate, as well as degenerative conditions. Typically, these factors complicate the treatment of onychomycosis, especially if the patient has a combined pathology of the nail (for example, mycosis against the background of psoriasis).
In the pathogenesis of onychomycosis, a significant role belongs to the pathology of carbohydrate metabolism, endocrine and neurological changes, and disorders of the immune system. The disease often develops against the background of long-term use of medications that suppress the body's defenses (glucocorticoids, cytostatics, broad-spectrum antibiotics). According to the literature [8, 23], onychomycosis is registered in 40% of cases in people over 65 years of age who have age-related immunosuppression.
The vast majority of cases of onychomycosis are characterized by a chronic persistent course. It is believed that when Tr. rubrum
this is due to the inability to develop a specific immune response against the fungus due to the activity of special T-suppressor cells. Suppression of the cellular and lack of inflammatory response to the pathogen may also be due to its predominant localization in the stratum corneum of the epidermis and nail plates.
The literature provides numerous data on the comparative effectiveness of various methods of treating onychomycosis: local use of antimycotics in the form of ointments, patches, varnishes, surgical removal of the nail plate followed by treatment of the nail bed with antifungal drugs, as well as systemic antimycotics [1, 2, 12, 28]. An analysis of publications on the problem indicates that in world medical practice there is still no ideal remedy and method for treating onychomycosis that would be suitable for all patients without exception, would not give any side effects and would provide a 100% clinical and etiological cure of the patient in a short time. Therefore, today the search and comprehensive study of new effective antimycotics that meet these requirements is justified.
A representative of the class of allylamines, chemical compounds included in the arsenal of the most effective treatments for dermatomycosis, is terbinafine (Lamisil, created in 1982 by the Swiss company Novartis).
To date, after multilateral clinical trials, Lamisil has become firmly established in the practice of treating patients with mycoses [11, 22, 29, 31]
This is a drug with a wide spectrum of antifungal activity [16, 34]. At therapeutic concentrations, it has a direct fungicidal effect on dermatophytes, yeasts and molds, both orally and when applied topically. The site of application of the drug is the cytoplasmic membrane of the fungal cell. The advantage of terbinafine is its action in the early stages of sterol metabolism, at the level of the squalene epoxidase cycle. By inhibiting the enzyme squalene epoxidase, terbinafine inhibits the formation of ergosterol, the main component of the cell wall of fungi, preventing their further reproduction [15, 19, 22].
The drug has a dual mechanism of action: fungicidal and fungistatic, with the fungicidal effect being the main one. It is caused by the accumulation of squalene in the fungal cell, which, like a kind of lipid sponge, extracts lipid components from membranes. Lipid granules accumulating inside the cell, gradually increasing in volume, rupture the defective cytoplasmic membranes, leading the fungal cell to death [11, 17]. The fungistatic effect is due to damage to the membranes of the fungal cell due to the suppression of ergosterol synthesis, which leads to the loss of the fungal cell’s ability to develop - it only survives.
The predominant fungicidal effect of Lamisil, which distinguishes it from previously created systemic antimycotics, determines the higher effectiveness of the drug, as well as a lower number of relapses when used, due to which treatment is cheaper than other systemic antimycotics [9, 14].
The drug is well absorbed from the gastrointestinal tract: 2 hours after a single dose of 250 mg, its plasma concentration is 0.8–1.5 mg/ml. Due to its lipophilicity, it accumulates in the dermis, epidermis, and adipose tissue, from where it is slowly absorbed into the blood [19]. Terbinafine also penetrates the secretions of the sebaceous glands, which leads to the creation of the highest possible concentrations in the hair follicles, hair and skin rich in sebaceous glands. The drug is detected in the stratum corneum of the epidermis within a few hours after oral administration, which is achieved by its excretion by the sebaceous glands and, to a lesser extent, by passive diffusion [11, 17]. Terbinafine is characterized by long-term persistence in the blood due to a continuous dosage regimen, which is an important component of its pronounced effectiveness. In the distal parts of the nails, its concentration remains for 48 weeks after the end of the course of therapy, which causes a positive fungicidal effect and makes it possible to cure onychomycosis without removing the nail plates [19, 29]. Biotransformation leads to the formation of metabolites that do not have antimycotic activity, which are excreted from the body mainly in the urine [9].
The distribution features of terbinafine include its lymphatic transport due to its lipophilicity and association with chylomicrons. Through the lymphatic vessels, terbinafine directly reaches infiltrative-suppurative and abscessed lesions complicated by lymphangitis. This nature of terbinafine transport, combined with its antibacterial properties, comparable to those of gentamicin, leads to a fairly rapid resolution of complicated forms of dermatophytosis [23].
The drug does not suppress liver enzymes, has little binding to cytochrome p-450, and as a result has virtually no effect on the metabolism of medications, i.e., the risk of drug interactions is minimal. This is an important advantage of the drug, since it ensures safety in the treatment of patients receiving concomitant therapy for intercurrent diseases (diabetes mellitus, diseases of the cardiovascular, nervous systems, etc.) [21, 31]. The bioavailability of terbinafine does not change as a result of co-administration with other drugs and food. At a concentration 5 times higher than the therapeutic one, terbinafine does not inhibit chemotaxis, phagocytosis and metabolic activity of leukocytes, i.e. does not have an immunosuppressive effect [34]. Moreover, the work of Barbareschi, 1993 [13] showed that in vitro incubation of polymorphonuclear neutrophils with terbinafine leads to an increase in their activity [13].
Lamisil has an excellent safety profile [20, 36, 39, 40]. Its safety is comparable to placebo [29]. According to data presented by a number of authors [16,27], based on the analysis of case histories of 25,884 patients taking tablets, side effects that occurred when taking Lamisil are extremely rare and relate mainly to mild phenomena from the gastrointestinal tract (Table 1 ).
All noted properties of the drug contributed to its rapid introduction into clinical practice in more than 80 countries around the world [17, 21, 25, 27]. The data from foreign researchers presented in the table indicate the high effectiveness of Lamisil in the treatment of onychomycosis. Many domestic scientists have also noted the positive experience of treatment with Lamisil, demonstrating very impressive results.
So, according to A.A. Kubanova [4], positive clinical effects and microbiological cure were noted in 94.4% of patients. The results of Yu.V. are indicative. Sergeev [9], which demonstrates clinical and mycological cure in 82% of patients. Data from N.S. Potekaeva [8] indicate the high effectiveness of Lamisil: mycological cure was obtained in 96% of patients (see Table 2). V.M. Leshchenko and G.M. Leshchenko [6] assess the results of treatment of patients with onychomycosis of the feet and hands, combined with lesions of smooth skin, as positive. At the end of the traditional course of treatment (250 mg once for 12 weeks), clinical recovery was observed in 96% of patients. The drug was well tolerated. In 478 patients after treatment with Lamisil, no fungi were microscopically detected in the material from the nail plates, and 6 months after the start of treatment, clinical and microbiological cure was stated in all of them.
According to V.A. Molochkov [5], in the treatment of patients with onychomycosis, the use of Lamisil turned out to be highly effective, positive clinical effects and microbiological cure were noted in 95.2% of patients. Interesting data on the use of Lamisil in patients with diabetes mellitus were obtained by S.A. Burova [3]. The presented long-term results (6 months after the end of treatment with Lamisil) indicate that clinical recovery is observed in 85.7% of patients, microbiological sanitation in 89.3%. HE. Pozdnyakova demonstrates the effectiveness and safety of Lamisil in 48 patients with chronic liver diseases without exacerbation [8] in the compensated and subcompensated stages: after completion of therapy, mycological cure was observed in 100% of patients. The information on the use of Lamisil in immunodeficiency states is encouraging, given that the number of these patients is increasing every year. To date, 82 cases of the use of Lamisil tablets for immunodeficiencies have been described: AIDS [26, 37, 38], Down syndrome, after organ transplantation [18, 24, 27].
Yu.K. Skripkin and Zh.V. Stepanova [10], summarizing the experience of domestic and foreign researchers, once again emphasized the effectiveness and safety of Lamisil, good tolerability, and convenient application regimen. The effectiveness of treatment, according to their data, was 94%.
We observed 32 patients with onychomycosis of the feet (18 men, 14 women) aged from 25 to 55 years with a disease duration of 1 to 15 years. Concomitant diseases were identified in 28% of patients, including gastritis, colitis, thyroid dysfunction; 50% of patients considered themselves practically healthy. Predisposing factors determining the development of the mycotic process were identified in 100% of patients. Thus, 26 patients were regular visitors to swimming pools, 18 to saunas; 14 patients were involved in professional sports, which is associated with permanent trauma to the nail plates. The diagnosis of onychomycosis was established in each case on the basis of the clinical picture of the disease and the detection of fungi during microscopic examination of the affected nail plates. In 20 patients, in addition to onychomycosis (damage from 1 to 16 nail plates), there was damage to the skin of the corresponding localization: feet and hands (4 patients), smooth skin of the torso (6 patients), large folds (10 patients). According to the clinical picture, the nail plates were affected mainly by a mixed type, i.e. in the same patient on different fingers they were changed in a hypertrophic, normotrophic type with symptoms of onycholysis and the area of nail damage from marginal (5–10%) to total, involving the skin of the feet and palms in the process with skin changes characteristic of rubrophytosis exaggeration of skin furrows, fine-plate (floury) peeling, hyperkeratosis, maceration of the epidermis, cracks in the interdigital folds of the skin of the feet.
Patients received Lamisil orally at a dose of 250 mg per day for 12 weeks, and during therapy, a general blood test, urine test, and liver function tests were examined. In each case, shoes were treated at home with a 25% formaldehyde solution or a 0.5% chlorhexidine digluconate solution twice with an interval of 1 month. In order to increase the intensity of growth of the nail plate, 5 patients were prescribed phytin and zinc-containing vitamin complexes orally.
The patients tolerated the treatment well. Among the side effects, 1 patient experienced a temporary loss of taste: this phenomenon resolved on its own without requiring discontinuation of the drug.
Control microscopic examination was carried out 3, 4, 6 months after the start of treatment, and 6 months after the end of therapy, in 100% of cases the results of microscopic examination were negative. In 4 patients, despite the absence of fungi on microscopic examination, the nail plates remained dystrophically changed, and in each case they had a history of trauma to the corresponding nail.
As a result of treatment, skin rashes, especially in large folds, resolved within 2–3 weeks. Affected nails with an affected area of up to 60% on the feet were replaced with visually healthy ones after 5–6 months, i.e. 3–4 months after stopping taking Lamisil. No relapses of the disease were detected (9–14 months of follow-up).
Thus, based on literature data and the results of our own research, it can be argued that the advent of Lamisil has led to a significant improvement in the prognosis of patients suffering from onychomycosis, which was previously difficult to treat and characterized by a persistent course. Lamisil therapy has changed the view that onychomycosis is difficult to cure: the drug is highly effective, providing a high cure rate in a shorter period of time than previous generations of antimycotics. The fungicidal effect, active penetration of Lamisil into keratin and its long-term preservation in the nail plate, combined with the relative rarity of serious undesirable effects and relapses, have made the drug the drug of choice in the treatment of fungal infections of nails and smooth skin. It should be noted that it is currently included in the list of drugs for preferential treatment of disabled people and pensioners at the expense of insurance companies.
The list of references can be found on the website https://www.rmj.ru
Terbinafine–
Lamisil (trade name)
(Novartis Pharma)
References:
1. Afanasyev D.B. “Complex outpatient treatment of onychomycosis using biologically active dressings”, abstract of the dissertation of Candidate of Medical Sciences. Sciences, M., 1996, 23.
2. Bormotov V.Yu. “Outpatient treatment of patients with onychomycosis caused by red trichophyton”, Abstract of the dissertation of candidate of medical sciences., M., 1983; 19.
3. Burova S.A., Talalaeva S.M., “Long-term results of treatment of onychomycosis in patients with diabetes mellitus” “Russian Journal of Skin and Venereal Diseases” 2000, No. 5, pp. 31–33.
4. Kubanova A.A., Sukolin G.I., Yusuf M., Yazdy M.Sh. “Bulletin of Dermatology and Venereology” 1995, No. 6, pp. 42–43 “The use of Lamisil in mycological practice.”
5. Kurcheva O.P., Molochkov V.A. “Lamisil is an effective treatment for onychomycosis” “Russian Journal of Skin and Venereal Diseases” 1998, No. 1, 47–49.
6. Leshchenko V.M., Leshchenko G.M. “Treatment of onchomycosis with Lamisil” “Bulletin of Dermatology and Venereology” 1998, No. 2, pp. 61–64.
7. Lykova S. G., Pozdnyakova O. P. “Bulletin of Dermatology and Venereology”, 2000, No. 4
8. Potekaev N. S. “Bulletin of Dermatology and Venereology”, 1999, No. 5
9. Sergeev Yu.V., Potekaev N.S., Leshchenko V.M., Larionova V.N. “Bulletin of Dermatology and Venereology” 1995 No. 5 pp. 54–56 “Lamisil: improving the treatment of onychomycosis caused by dermatophytes.”
10. Skripkin Yu.K., Stepanova Zh.V. “Bulletin of Dermatology and Venereology” No. 6, 1999. 11 11. Alpsoy E., Yilmanz E., Basaran E. Intermettent therapy with terbinafine for dermatophyte toe onychomycosis a new approach.– J. Dermatol, 1996, 23:259–262
12. Back DJ, Tjia JF, Abel SM Azoles, allylamines and drug metabolism. Br. J. Dermatol. 1992; 126(Suppl. 33): 14–18.
13. Barbareschi M, Vago, Colombo D., Bevilacqua M. mycoses 36: 405–9 (1993), (LAS 360)
14. Clayton JM In vitro activity of terbinafine, Clin Ep.Dermatol. 1989; 14: 101–103.
15.Clayton JM, Hay KJ, Bnt. J. Dermatol. 1994 vol.130 suppl.43, p.9–11.
16. De Cnyper “Long-term outcome of onychomycosis treatments” Cl. Dermal, 1998; TZ
17. Einarson TR, Lupta AK, Shear NH, Arikian S. Clinical and economic factors in the treatment of onychomy costs. Pharmacoeconomics 1996: 307–320.
18. EnsenP. et al. ActaDermato-Venereologica76: 280–1 (1996)
19.Faergemann J., Lehender H., Milleriouv L. Levels of terbinafine in plasma, stratum comeum, dermis–epidermis (without stratum comeum), sebum, hair and nails during and after 250 mg terbinafme orally once daily for 7 and 14 days .– Clin. Exp. Dermatol. 19:121–6.
20. Hall M., Monka C., Krupp P., O'Sullivan Safety of oral terbinafme. Results of a Postmarketing Surveillance study in 25884 patients.–Archives of Dermal, 1997, v. 1213–1218.
21. Goodfield MJD, Br. J. Dermatol. 1992; 126 suppl. 33–35
22. Jones T.S. Overview of the use of terbinafme (Lamisil) in children. Br. J. of Dermatology; 1995; 132:683–689.
23. Lamisil (terbinafine hydrochloride tablets). Physicians' Desk Reference. 51 st col Montvale, NJ: Medical Economics; 1997.2334–95.
24. La Placa M et al. J. Dermatol. Tream 6:51–2(1995) (LAS 446)
25. Matsumoto T., Januma H., Kaneko S., Jakasu H., Nishiyama S. Clinical and pharmacokinetic investigations of oral terbinafme in patients with tinea unguium. – Mycoses 1995; 38: 135–144.
26. Nandwani R. et al. Br J Dermatol. 134 (suppl. 46): 22–24 (1996) (LAS 563)
27. Norden J et al. Scand J Infect Lis 23: 377–82 (1991) (LAS 188) 2 8. Onychomy costs and terbinafine. Lancet 1990; 335:636
29. Polak A., Handbook of Experimental Pharmacology; Chemotherapy of Fungal Diseases 1990, p.96–153.
30. Roberts DT et al J. Amer. Assoc. Dermatol. 1994; 31 (suppl.2) 578–581.
31. Roberts DT Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey.– Br. J. Dermatol. 1992; 126 (Suppl. 39): 23–7
32. Roberts Dabriol. Abstracts of the 19th world congress of dermatology, 1997, Sydney, Australia
33. Rynder NS The mechanism of action of terbinafine. Clin. Exp. Dermatol. 1989; 14:98–100.
34.Shauder M. Mycoses 31:259–67 (1988) (LAS 62)
35.Singer MJ et al, J.Amer.Acad Dermatol. 1997, 37; 765–771.
36.Suhonen R. and Neuvonen P., Rev.Contemp.Pharmacother, 1997; 8; 373–386.
37. Villars V et al. Br J Dermatol 126(suppl 39):61–9 (1992) (LAS 208)
38. Velthuis PJ et al. Br J Dermatol 144–6(1995) (LAS 465).
39. Williams and Hall M. “A review of the Safety of oral terbinafine (Lamisil) when used with oral coutraceptives and in pregnancy in a Postmarketing Surveillance Study.”– Book of abstracts, poster No. 81, Sing., 1988, 12– 20.06, pm 18.
40. Williams T., Lanslandt J. and Jones T. A review of the efficacy and tolerability of terbinafine in children. Poster presented at the 8th International Congress Pediatric Dermatology, 1998.
Lamisil tab 250mg No. 14
Compound
Active substance: terbinafine (in the form of hydrochloride) 250 mg.
Excipients: magnesium stearate, colloidal anhydrous silicon dioxide, methylhydroxypropylcellulose, microcrystalline cellulose, sodium starch glycolate.
Indications for use
- Onychomycosis caused by dermatophyte fungi;
- mycoses of the scalp;
- fungal infections of the skin - treatment of dermatomycosis of the trunk, legs, feet, as well as yeast infections of the skin caused by fungi of the genus Candida (for example, Candida albicans) - in cases where the localization, severity or prevalence of the infection determine the advisability of oral therapy.
Unlike topical Lamisil, oral Lamisil is not effective for tinea versicolor.
Contraindications
- Hypersensitivity to the components of the drug.
It is not recommended to prescribe Lamisil to patients with chronic or active liver disease. Before prescribing Lamisil tablets, it is necessary to determine whether the patient has pre-existing liver disease. Hepatotoxicity can occur in patients with or without pre-existing liver disease. Patients prescribed Lamisil should be warned that it is necessary to immediately inform the attending physician if symptoms such as persistent nausea, lack of appetite, fatigue, vomiting, pain in the right hypochondrium, jaundice, dark urine or light-colored stool occur while taking the drug. . If such symptoms occur, you should immediately stop taking the drug and conduct a liver function test.
Since the use of the drug in patients with impaired renal function (creatinine clearance <50 ml/min or serum creatinine concentration >300 µmol/l) has not been sufficiently studied, Lamisil is not recommended for use in this category of patients.
Directions for use and doses
The duration of treatment depends on the indication and severity of the disease.
There are no data on the use of the drug in children under 2 years of age (whose body weight is usually less than 12 kg). In children over 2 years of age, Lamisil for oral administration is well tolerated.
| Body mass | Dose |
| less than 20 kg | 62.5 mg |
| from 20 to 40 kg | 125 mg |
| more than 40 kg | 250 mg |
Adults are prescribed 250 mg 1 time/day.
The recommended duration of treatment for tinea pedis (interdigital, plantar or sock-type) is 2-6 weeks; for dermatomycosis of the trunk, legs - 2-4 weeks; for skin candidiasis - 2-4 weeks.
Complete disappearance of the manifestations of infection and complaints associated with it can occur only a few weeks after mycological cure.
The recommended duration of treatment for mycosis of the scalp is 4 weeks. Mycoses of the scalp are observed mainly in children.
For onychomycosis, the duration of effective treatment in most patients is from 6 to 12 weeks. For onychomycosis of the hands, in most cases, 6 weeks of treatment is sufficient. For onychomycosis of the feet, in most cases, 12 weeks of treatment is sufficient. Some patients who have a reduced rate of nail growth may require longer treatment. The optimal clinical effect is observed several months after mycological cure and cessation of therapy. This is determined by the period of time required for a healthy nail to grow back.
There is no reason to assume that elderly patients require dosage adjustments or that they experience adverse reactions that differ from those of younger patients. When using the drug in tablet form in this age group, the possibility of concomitant liver or kidney dysfunction should be taken into account.
Storage conditions
The drug should be protected from exposure to light. Store at a temperature not exceeding 30°C, out of the reach of children.
Best before date
5 years. Should not be used after the expiration date marked on the package.
special instructions
Terbinafine has been shown to inhibit metabolism mediated by the CYP2D6 enzyme. Therefore, it is necessary to constantly monitor patients receiving concomitant treatment with Lamisil with drugs that are predominantly metabolized with the participation of this enzyme (such as tricyclic antidepressants, beta-blockers, selective serotonin reuptake inhibitors, class 1C antiarrhythmic drugs and MAO inhibitors if used simultaneously the drug has a small range of therapeutic concentrations.
Description
Antifungal agent.
Use in children
There are no data on the use of the drug in children under 2 years of age (whose body weight is usually less than 12 kg). In children over 2 years of age, Lamisil for oral administration is well tolerated.
| Body mass | Dose |
| less than 20 kg | 62.5 mg |
| from 20 to 40 kg | 125 mg |
| more than 40 kg | 250 mg |
Pharmacodynamics
Antifungal drug.
Terbinafine is an allylamine that has a broad spectrum of action against fungi that cause diseases of the skin, hair and nails, incl. dermatophytes such as Trichophyton (for example, Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton verrucosum, Trichophyton tonsurans, Trichophyton violaceum), Microsporum (for example, Microsporum canis), Epidermophyton floccosum, as well as yeasts of the genus Candida (for example, Candida albicans) and Pityrosporum. In low concentrations, terbinafine has a fungicidal effect against dermatophytes, molds and some dimorphic fungi. Activity against yeast fungi, depending on their type, can be fungicidal or fungistatic.
Terbinafine specifically inhibits the early stage of sterol biosynthesis in the fungal cell. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. Terbinafine works by inhibiting the enzyme squalene epoxidase in the cell membrane of the fungus. This enzyme does not belong to the cytochrome P450 system.
When Lamisil is administered orally, concentrations of the drug are created in the skin, hair and nails, providing a fungicidal effect.
Side effects
Lamisil is generally well tolerated, side effects are usually mild to moderate and transient. The following are adverse events that were observed during clinical trials or after the drug was marketed.
When assessing the frequency of side effects, the following gradations were used: very often (≥1/10), often (≥1/100, <1/10), sometimes (≥1/1000, <1/100), rarely (≥1/ 10,000, <1/100), very rare (<1/10,000), including isolated reports.
From the hematopoietic system: very rarely - neutropenia, agranulocytosis, thrombocytopenia, pancytopenia. In very rare cases, when using the drug, the development of qualitative or quantitative changes in blood cells (neutropenia, agranulocytosis, thrombocytopenia, pancytopenia) was observed. If qualitative or quantitative changes in blood cells develop, the cause of the disturbances should be established and consideration should be given to reducing the dose of the drug or, if necessary, discontinuing therapy with Lamisil.
From the immune system: very rarely - anaphylactoid reactions (including angioedema), cutaneous and systemic lupus erythematosus.
From the nervous system: often - headache; sometimes - disturbances in taste, including their loss (usually recovery occurs within a few weeks after stopping treatment). There are isolated reports of cases of long-term disturbances in taste. In some cases, while taking the drug, there was a decrease in food consumption, which leads to a significant decrease in body weight. Dizziness, paresthesia, and hypoesthesia were very rarely observed.
From the hepatobiliary system: rarely - hepatobiliary dysfunction (mainly cholestatic in nature), including very rare cases of severe liver failure (some fatal or requiring liver transplantation). In most cases where liver failure developed, patients had serious concomitant systemic diseases and the causal relationship of liver failure with Lamisil was questionable.
From the digestive system: very often - a feeling of fullness in the stomach, loss of appetite, dyspepsia, nausea, mild abdominal pain, diarrhea.
Dermatological reactions: very often - mild skin reactions (rash, urticaria); very rarely - serious skin reactions (including Stevens-Johnson syndrome), psoriasis-like skin rashes or exacerbation of psoriasis. Very rarely, cases of hair loss have been reported, although the cause-and-effect relationship of this phenomenon with taking the drug has not been established. If a progressive skin rash develops, treatment with Lamisil should be discontinued.
From the musculoskeletal system: very often - arthralgia, myalgia.
Other: very rarely - feeling of fatigue.
Use during pregnancy and breastfeeding
Data from experimental studies do not suggest the presence of adverse effects on fertility and toxic effects on the fetus. Since clinical experience with Lamisil during pregnancy is very limited, the drug should not be used during pregnancy unless the expected benefit of therapy outweighs the potential risk.
Terbinafine is excreted in breast milk, so women receiving Lamisil by mouth should not breast-feed.
Interaction
Effect of other drugs on terbinafine
Plasma clearance of terbinafine can be accelerated by drugs that are metabolic inducers and suppressed by cytochrome P450 inhibitors. If it is necessary to use the above drugs and Lamisil simultaneously, an appropriate adjustment of the dosage regimen of the latter may be required.
Cimetidine may enhance the effect of terbinafine or increase its plasma concentration. Cimetidine reduces the clearance of terbinafine by 33%.
Rifampicin may weaken the effect of terbinafine or reduce its plasma concentration. Rifampin increases the clearance of terbinafine by 100%.
Effect of terbinafine on other drugs
Results from in vitro and healthy volunteer studies indicate that terbinafine has little potential to inhibit or enhance the clearance of most drugs metabolized by the cytochrome P450 system (e.g., terfenadine, triazolam, tolbutamide, or oral contraceptives), except those which are metabolized with the participation of CYP2D6.
Terbinafine does not affect the clearance of antipyrine or digoxin.
There have been reports of several cases of menstrual irregularities in patients taking Lamisil in conjunction with oral contraceptives, although the frequency of these disorders does not exceed the average frequency of such disorders in patients taking only oral contraceptives.
Terbinafine may enhance the effects of caffeine or increase its plasma concentration. Terbinafine reduces the clearance of caffeine when administered intravenously by 19%.
Terbinafine has been shown to inhibit CYP2D6-mediated metabolism in in vivo and in vitro studies. These data may be clinically significant for those drugs that are predominantly metabolized by this enzyme: tricyclic antidepressants, beta-blockers, selective serotonin reuptake inhibitors, class 1A, 1B, 1C antiarrhythmic drugs and MAO type B inhibitors, if the drug used At the same time, the drug has a small range of therapeutic concentrations.
Terbinafine reduces the clearance of desipramine by 82%.
Terbinafine may weaken the effect of cyclosporine and reduce its plasma concentration. Terbinafine increases the clearance of cyclosporine by 15%.
Overdose
Symptoms: there are reports of several cases of overdose (the dose taken was up to 5 g), in which headache, nausea, pain in the epigastric region and dizziness were noted.
Treatment: measures to eliminate the drug, primarily by prescribing activated charcoal and gastric lavage, and, if necessary, using symptomatic maintenance therapy.
Impact on the ability to drive vehicles and operate machinery
The effect of Lamisil on the ability to drive vehicles and operate machinery has not been studied. If dizziness develops during drug therapy, patients should not drive vehicles and/or operate machinery.