Exudative erythema multiforme, clinical, immunological and therapeutic features
Exudative erythema multiforme (EME) is an acute, often recurrent disease of the skin and mucous membranes (O. L. Ivanov, 1997). The etiological factors of MEE are varied. Patients with this diagnosis have a trigger factor that “turns on” the mechanism of the immune hypersensitivity reaction. Triggering factors are divided into two groups: allergens of medicinal, food, etc. nature, causing a toxic-allergic type of dermatosis, and infectious - viruses, bacteria, protozoa, which cause the infectious-allergic form of MEE.
Clinical manifestations of the disease
The picture of the disease includes a characteristic, often papular rash, which acquires in the process of evolution due to a centrifugal increase in elements and resolution of “two-color spots.” When they appear, the elements have a diameter of 2-3 mm and in one or two days (sometimes faster or slower), they increase to 1-3 cm, less often to a larger size. The name “multiform” is justified by the fact that patients may have spots, pustules, blisters in varying quantities; elements of the “palpable purpura” type are less common (in our practice, we observed two such patients, they had hemorrhages along with typical elements ).
In the case of a monomorphic blistering rash, in the absence or a small number of typical “targets”, the diagnosis of MEE can be difficult. In a 54-year-old patient, we observed a monomorphic blistering rash, represented by six large (up to 7 cm in diameter) weak blisters on the lateral surfaces of the body. The general condition was not disturbed; acantholysis and eosinophilia were not noted in the areas of the rash. There were already several such exacerbations in the anamnesis; the appearance of blisters was preceded by pain at the site of a long-standing scar in the lumbar region left after herpes zoster. MEE was diagnosed against the background of reactivation of the varicella-zoster virus. Taking acyclovir helped to stop the manifestations of the disease. Subsequently, the patient reported that she constantly takes acyclovir, and the rash no longer bothers her.
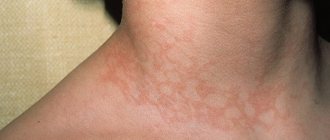
Localization of the rash with MEE is the face, mucous membranes, extensor surface of the extremities, backs of the hands and feet, palms, soles, torso. A number of studies indicate that MEE does not affect the scalp, however, we observed in one of the patients, along with a typical papular rash, blisters that were also localized on the scalp. In the parietal and occipital region there were four elements that were covered with crusts at the time of examination. Subsequently, when removing the crusts, epithelized round erosions with a diameter of 0.8-1.5 cm were visible. The location of the rash is always symmetrical.
With a diverse “palette” of color elements in different patients, a pink or purple tint is almost always observed, probably associated with the predominance of lymphocytes. As an analogue, we can cite lichen planus, which is characterized by a lilac-colored rash and in the histological picture there is mainly a lymphoid infiltrate in the dermis, closely adjacent to the epidermis. We consulted a patient with a periodically recurring monomorphic papular rash in the elbow area that did not disappear for two to three weeks; the elements did not merge, had a pinkish tint, and a regular flattened shape. Typical “targets” were absent, but the pink tint of the elements, as well as the type of dermatosis, made it possible to diagnose MEE. The survey revealed that relapses develop one week after labial herpes. Accordingly, the patients were diagnosed with herpes-associated exudative erythema.
The course of MEE is acute, and there is a tendency to recur with varying frequency. Rarely, recurrent forms resolve without treatment or after minimal treatment. We consulted a patient whose exacerbations of MEE were seasonal and were confined to the spring and autumn institute sessions. When collecting anamnesis, the patient said that the rash appears in full within several days, then begins to gradually regress. From time to time he used topical steroids, which somewhat accelerated the regression of the rash. In the majority of our patients, MEE recurred frequently or permanently existed in the form of a small amount (up to 5% of the body surface without damage to the mucous membranes) of rashes that did not cause subjective sensations; old elements regressed and new ones constantly appeared. For example, in a 60-year-old patient, five or six “target”-type elements were constantly present in the area of the back of the hands and forearms; the old ones regressed, leaving persistent hyperpigmentation, and slowly increasing new ones appeared. There were no subjective sensations, but localization in open areas of the body caused constant psychological discomfort. In another case, a 30-year-old man had a constantly increasing frequency of exacerbations and an increasing volume of rashes. On average, a month after therapy in the hospital, a new exacerbation developed. In these, as well as in other patients with frequent recurrence of dermatosis, spontaneous regression of the rash was not observed.
Pathogenetic and immunological aspects
Exudative erythema multiforme refers to diseases that have characteristic clinical manifestations, but are caused by different reasons (their composition will be discussed below). The constant symptoms characteristic of this dermatosis are a natural consequence of the unity of pathogenetic mechanisms, which at a certain stage occur in the same way in all forms of MEE. What is their morphological substrate? It is described primarily as a lymphocytic infiltrate around the vessels, with a small admixture of eosinophils or neutrophils. This rapidly accumulating infiltrate has the appearance of blue-pink papules. In the basal layer, intra- and extracellular edema is observed, the epidermis can peel off to form a bubble, the covering of which is formed by all layers of the epidermis; Accordingly, the tire can remain intact for one or two days, even under clothing or with a large bubble diameter. Extravasation may occur, externally manifesting as hemorrhagic elements. In general, since the clinical picture of MEE is quite typical, the need for a biopsy is rare. Pathohistological examination can be a serious aid in diagnosis if the clinical picture of MEE is represented mainly by blisters. Imprint smears make it possible to exclude acantholysis and, accordingly, pemphigus, and a small number of eosinophils in the cystic fluid reduces the likelihood of the presence of dermatitis herpetiformis and bullous pemphigoid.
From the point of view of allergology, MEE is a mixed reaction, having features of both immediate (IHT) and delayed (DTH) hypersensitivity. Features of HNT in MEE: an increase in total IgE (reagin antibodies) in almost all patients and the predominance of Th2 type cells among T helper cells (19), the presence of CD8+ cytotoxic lymphocytes in the infiltrate (3.9), which makes it similar to allergic contact dermatitis. Symptoms of immune complex pathology in MEE: deposition of IgM and the C3 component of complement in the areas of MEE rashes and detection in the blood of IC with the herpes simplex virus, one of the triggers of MEE [12, 17]. The fact that MEE cannot fit into the “Procrustean bed” of one of the classical allergic reactions, the classification of which was created back in 1969, is largely explained by the constant emergence of new data that makes it possible to divide allergies into an increasing number of types. The mechanism of development of MEE deserves detailed study; we will consider it below using the example of herpes-associated exudative erythema.
In conclusion, it can be noted that in any case, MEE is a manifestation of a shift in the adaptive mechanisms of the immune response towards hypersensitivity, i.e., it is an allergic reaction, regardless of the causative factor.
Predisposition and trigger factors for the development of MEE
For the development of exudative erythema, the patient must have hypersensitivity, which objectively finds expression in an almost total increase in IgE in patients. In the case of MEE, which has developed as a result of the administration of medications, the process follows the type of toxicdermia; accordingly, a strict distinction between the diagnosis “toxidermia” and the “toxic-allergic form of MEE” does not make sense and does not affect the volume and composition of the therapy, which is determined mainly by the severity of the clinical manifestations. Drug allergies are easier to identify than other types of allergic reactions. But with a general atopic predisposition of the body, a reaction can occur to food or pollen allergens; here the relationship is more difficult to identify, since it is not always recognized by the patient himself. However, it is worth trying to determine this relationship, since this will allow you to narrow the range of treatment measures (for example, exclude antibiotics, which are usually prescribed if the causes of MEE are not established). The following case can be cited as an example. A young man with multiple target-type rashes consulted a doctor. The diagnosis did not cause any difficulties, but the cause of the disease remained unclear. When collecting anamnesis, it turned out that the patient has a pronounced atopic background, suffers from bronchial asthma, suffered angioedema twice in childhood, and also has pollen and food allergies. In particular, after eating spicy food, there is always a short-term dilution of the stool. On the eve of the appearance of the rash, the patient tried a new, very spicy dish in a restaurant. Accordingly, in this case, MEE has a toxic-allergic form and requires measures aimed at eliminating the antigen. In general, the toxic-allergic variety of MEE is characterized by clinical and diagnostic features, which will be discussed below. The proportion of the toxic-allergic form of MEE is small; according to various sources, it does not exceed 20%. It is possible that such a low incidence of development of this form of MEE is due to the fact that such a diagnosis is made only if the fact of taking medications is revealed, while, for example, food allergies in adults often manifest themselves in the form of skin lesions. This once again demonstrates the importance of taking anamnesis. With frequent recurrence of MEE and the impossibility of identifying a trigger, especially with a history of allergic reactions, it may be recommended to keep a food diary to identify food allergies.
The infectious-allergic form of dermatosis is more common and creates more therapeutic difficulties. There are many known bacterial and viral agents that provoke the development of MEE. As a rule, these are pathogens that form a focus of chronic persistence in the body with periodic exacerbations, which contributes to sensitization to the infection. The role of bacterial allergy has repeatedly found objective confirmation in various studies [2, 13, 14, 15]. The localization of the outbreak can be any. The presence of a chronic infection in the body, which is already a consequence of certain defects of the immune system, aggravates them and can cause damage to the protective anti-infective response, including promoting the production of mediators of the immune response, among the effectors of which there are clones of cells that form hypersensitivity reactions. In the patients we observed, we found foci of infection more often in the nasopharynx, paranasal sinuses and respiratory system, less often it had a urogenital localization. As a rule, the source of infection cannot be determined during the first conversation with the patient; in most cases, the search for infection requires examination by related specialists, as well as laboratory and instrumental examination. A newly developed acute infection can also provoke MEE, but in this case it occurs after one to two weeks. At the same time, MEE for exacerbation of a chronic lesion can manifest itself in the first days of its activation.
The pathology of the digestive tract, which is considered to be one of the reasons for the development of MEE, was also not obvious; in all patients, during questioning and examination, minimal symptoms of chronic colitis, biliary dyskinesia, more often of the hypomotor type, signs of chronic gastritis, dysbiosis of the intestinal microflora of the first - second degree. However, all patients had a reduced level of IgA, the final stage of synthesis of which occurs in the intestinal wall.
The impetus for the development of MEE can be stress in the broad sense of the word. In the patients we observed, the “push” factor was more often psychological stress and overwork at work or school (various types of tests, sessions), and less often - hypothermia. In one patient, MEE debuted after emotional stress (death of the father), and in another three patients after hyperinsolation. A young woman who contacted us developed MEE after orthostatic load (long walking or standing). The clear dominance of psychological stress as a trigger emphasizes the relevance of observing a rational work and rest regimen in patients with recurrent MEE, as a component of the prevention of exacerbations.
Thus, the background for the development of MEE is atopy, expressed in an increase in total IgE, persistence of chronic infection in the body, and a decrease in IgA, which is one of the representatives of the “first line of defense” of the skin and, to a greater extent, mucous membranes. The impetus for relapse of MEE is often stress or overwork.
Clinical features of MEE depending on the etiological agent
| Picture 1 |
| Figure 2 |
| Figure 3 |
| Figure 4 |
| Figure 5 |
| Figure 6 |
Given the general patterns of rash localization and characteristics of MEE elements, the etiology of the disease determines some of its features. We observed 14 patients with an infectious-allergic form of MEE (non-viral origin) and six with a toxic-allergic form. The toxicodermic variant was characterized by a greater prevalence of rash, damage to the trunk and proximal extremities, and involvement of the mucous membranes. The rashes were represented by foci of edematous hyperemia, had a bright red tint, a diameter of up to 10 cm, and tended to group into large irregularly shaped foci. At the same time, small “targets” with the appearance of a “target” characteristic of MEE were found along the periphery of large foci. Large figures resulting from the merger also began to resolve from the center (see Figure 1). Sometimes, against the background of large spots, small intraepidermal vesicles form due to spongiosis (see Figure 2). If the rashes of toxic-allergic MEE were limited in nature, then they were associated with oral intake of the antigen and tended to be localized on the oral and genital mucosa, which was due to the route of entry and elimination of the antigen (see Figures 3, 4). If the hands were affected, then the surface of the palms was more often involved in the process (see Figure 5), while infectious-allergic MEE was more characterized by damage to the back of the hands (see Figure 6). (When examining a typical element located on the back of the hand, it is clear that along the periphery of the “target” there are two small papules, which in the process of centrifugal spread can acquire a characteristic appearance.) In the case of a toxic-allergic form with a rash like “targets” in the center elements, bubbles more often developed, i.e., the “target-like appearance” was caused not by resolution from the center, but by the formation in the center of bubbles with transparent contents and a dense cover. An isomorphic reaction was observed in places of friction of clothing or shoes; the elements tended to merge with the formation of pseudo-single-chamber bubbles of irregular shape. However, the general condition of the patients did not suffer much.
The infectious-allergic form of the disease was characterized by all possible variants of clinical manifestations of MEE. The severity of the manifestation depended on the degree of sensitization of the body to the antigen and the background state of anti-infective immunity. In general, it can be noted that the infectious form of MEE was characterized by a smaller diameter of the lesions (up to 2 cm), a more “stagnant”, bluish tint of the rash and a tendency to localize on the extremities. We did not observe any trend toward mergers. In addition, in the infectious-allergic form, the genital mucosa was less often affected, in contrast to the toxic-allergic form.
The toxic-allergic form of MEE is characterized by more pronounced hyperemia, a tendency to merge lesions, frequent damage to the mucous membranes, including the genitals, a more pronounced epidermolytic component (bubbles), and an isomorphic reaction. The infectious-allergic form often manifests itself as small elements, more “stagnant” in color, without a tendency to merge, tending to be localized on the extremities and less often affecting the mucous membranes.
Herpes-associated erythema multiforme exudative
According to statistics, up to 80% of MEE is caused by the herpes simplex virus (HSV) [16], so we carried out work to assess the clinical and immunological parameters of this particular form, as the most common in practice. Among the patients with MEE who contacted us, 73% had herpes-associated exudative erythema (HAMEE).
It should be noted that we made the diagnosis of GAMEE on the basis of clinical data. These were typical manifestations of MEE, which developed within 12 hours to 12 days from the onset of relapse of herpes simplex (HS), which had a clinically obvious form - grouped vesicles on an edematous-erythematous background. Manifestations of GAMEE were characterized mainly by small foci - up to 2.5 cm in diameter, and were represented by the entire spectrum of elements possible in MEE. We observed a monomorphic rash only a few times: in two people, a papular rash represented by identical pink flattened papules with a diameter of 1-1.3 cm, grouped in the elbow areas in one patient and on the entire extensor surface of the arms, in small quantities on the legs and torso in the second patient. A 54-year-old woman was diagnosed with a bullous form - large blisters with a diameter of 2 to 7 cm, located on the lateral surface of the body. Basically, the rash with GAMEE was represented by flattened blue-pink papules up to 3 cm in diameter, which resolved from the center; in the center of the lesions, in some cases, blisters with a dense covering were formed, small pustules and isolated hemorrhagic lesions were encountered. A feature of GAMEE was the rare involvement of the genital area in the pathological process. The evolutionary dynamics of the rash as a whole did not differ from MEE. The recurrence rate of GAMEE was high, 5-12 times a year, which is probably due to the peculiarities of the etiological factor - PG, with violations of the antiviral component of immunity, prone to frequent recurrence.
GAMEE is prone to frequent recurrences, 5-12 times a year, is represented, most often, by solitary small papular elements of the “target” type, the prevalence of the rash varies. The genitals are rarely involved in the pathological process.
Immunological parameters of GAMEE
Along with the clinical features of GAMEE, its immunological parameters are of interest. An immunologically detected predisposition to GAMEE may, to some extent, indicate a predisposition to the development of herpes-associated diseases in patients with PG. After all, viral replication can change the gene apparatus of both resident cells, for example epidermocytes (in this case, this leads to lesions limited to the epidermis), and immunocompetent cells, then systemic diseases, for example SLE, can be observed. This assumption suggests the benefit of active therapy in patients with appropriate modifications. According to the same hypothesis, both the cell receptor apparatus and the enzyme or immunotransmitter (cytokine) profile can change [7].
GAMEE is characterized by an increase in IgE, a decrease in IgA, a decrease in the number of NK cells and g-interferon, a sharp increase in the spontaneous production of IL-4 and IL-6 over induced production, which is depleted, and a decrease in receptors for IL-2.
GAMEE is a mixed hypersensitivity reaction with an immune complex component of varying severity.
Therapy of exudative erythema multiforme
Treatment of this disease includes both stopping a relapse and preventing further exacerbations. It should be borne in mind that exudative erythema is an allergic reaction, regardless of the etiological factor. When a trigger is identified, appropriate measures are added.
When stopping a relapse, we took the type of dermatosis as a basis. If, upon initial treatment, the patient reports that he has frequent relapses and (or) profuse rashes, there are areas of necrosis in the center of the elements, mucous membranes are affected, the epidermolytic component is pronounced, we use a single injection of 2 ml of diprospan (injection solution including β-methasone in the form of disodium phosphate and dipripionate).
In our opinion, antibiotics should be prescribed only when a secondary infection occurs at the site of the rash or if there is a clear indication of an active focus of infection. In most cases, the effect of diprospan is quite sufficient to stop a relapse of MEE. In parallel, the trigger should be identified if there is a suspicion of a focus of chronic infection. The patient is referred for consultation to an otolaryngologist, therapist, or urologist; Swabs are taken from the patient to identify STIs. When collecting anamnesis in such patients, a positive effect from taking antibiotics without the use of diprospan and frequent colds are revealed. If the clinic corresponds to the toxic-allergic variant, the history contains indications of other allergic reactions, food and pollen allergies. In this case, additional measures should be taken aimed at the rapid elimination of the antigen: enterosorbents in case of food allergies, etc. In some cases, you can do without the use of diprospan. We are talking about patients for whom this exacerbation is not the first, goes away on its own or with the use of only local remedies - topical steroids, various types of mouth rinses (rotocan, chamomile infusion, sea buckthorn or rosehip oil, etc.). In this case, the main emphasis should be on identifying the trigger, and if it is impossible to detect it and rare relapses (once or twice a year), you can limit yourself to only stopping relapses using a minimum amount of therapeutic measures. Unreasonable use of various immunomodulators in case of rare recurrence of dermatosis can also upset the “precarious balance” and provoke an increase in exacerbations.
In general, it should be noted that diprospan is the optimal remedy if necessary to stop an exacerbation. Insufficient therapy with glucocorticosteroids in the early stages of drug allergies can be the main reason for the development of severe drug reactions and Lyell's syndrome in the future [6]; There are indications of the development of Lyell's syndrome after abortive attacks of MEE [1]. Considering that MEE is an allergic reaction regardless of the trigger, obviously this statement is to some extent true in relation to its other forms. We did not use diprospan in the presence of direct contraindications to the use of systemic steroids, as well as in “mild”, self-limiting course of dermatosis and in some cases for individual indications. In particular, we observed a patient with widespread damage to the skin (about 25% of the entire surface) and oral mucosa, with a pronounced epidermolytic component - blisters were formed in the center of all elements. There was a history of atopic background, including bronchial asthma, attacks of which the patient successfully stopped with Intal or Fenoterol. Toxic-allergic MEE (to a certain type of food) developed for the first time, however, various allergic reactions, also relieved with the help of Intal, were observed before. The general condition of the patients suffered to a very small extent. Despite the indications for the use of diprospan, we did not use it to influence the course of bronchial asthma, which did not require the use of steroids at the time of treatment. Therapy included oral mast cell membrane stabilizers, enterosorption, and topical steroids. Within a week, the rashes resolved, taking Intal was recommended in the future as preventive courses, and the patients were prescribed a hypoallergenic diet and consultation with an allergist.
Treatment of MEE includes stopping the relapse, often with the use of diprospan, which can be dispensed with in the case of a self-limiting course of dermatosis and exposure to the trigger, which varies depending on its nature.
GAMEE therapy
We observed 45 patients with GAMEE. We initially prescribed synthetic nucleosides to all patients, but the effect was observed only in a small number of patients. The majority, sooner or later, had to resort to prescribing diprospan. Having analyzed the situation, we came to the conclusion that nucleosides are effective in those patients whose herpes simplex has active manifestations at the time of development of GAMEE, i.e. is in the vesicular stage. Apparently, hypersensitivity in these patients develops at the height of virus replication, and interleukins do not yet have time to influence the entire spectrum of pathological action. Accordingly, by interrupting the replication of the virus, we block the further development of the process; in addition, an active viral process is a contraindication for the use of diprospan (although there are more and more reports about the parallel use of systemic steroids and nucleosides in GAMEE). In those patients whose body has regained control over HSV (crustosis stage of HSV is determined clinically), the adaptive mechanism has already fulfilled its function and entered the phase of uncontrolled hypersensitivity. Diprospan is indicated for these patients, and it is advisable to use synthetic nucleosides after the exacerbation has stopped, for the purpose of prevention. In general, GAMEE is easier to prevent than to treat, and long-term nucleoside supplementation has been repeatedly reported to be effective in preventing it. It remains to add that these drugs must be taken precisely on a continuous basis, since the disruption of the latency of the virus returns the immune system to its original level.
Subsequently, to prevent GAMEE, we used a herpetic polyvaccine: two courses of five injections at a dose of 0.1-0.2-0.3-0.3-0.3 with a two-week break between courses; revaccination was carried out after six months, and after a year - to consolidate the result. It is advisable to revaccinate if the first course was effective. If the first injection causes an exacerbation of the process, most likely it was prescribed too early. It is important to determine the moment when it is appropriate to start vaccination. We considered normalization of the levels of spontaneous and induced production of IL-4 and IL-6 as a criterion. During this process, which can be quite lengthy, we prescribed famciclovir 250 mg twice daily, or valacyclovir 500 mg twice daily, or acyclovir 200 mg four times daily. If, during the initial testing of the immune status, the levels of other cytokines involved in the formation of the inflammatory response, such as IL-1 or TNF, are elevated, normalization of the level of their production is necessary to begin vaccination, since an increase in these indicators increases the risk of post-vaccination complications.
The effectiveness of vaccination was 71%, which consisted of a reduction in the number of relapses of PG and, accordingly, a decrease in GAMEE by two to four times. Several patients were free of relapses during the entire follow-up period (one year). In general, the milder the course of GAMEE, the better the prognosis for vaccination, since severe skin damage is observed with the maximum component of hypersensitivity, and the longer it takes to normalize the cytokine profile. In conclusion, it can be recalled that the use of diprospan and vaccination have their contraindications, such as diabetes mellitus, peptic ulcer disease or glaucoma (for diprospan), therefore a careful history taking is necessary to identify them. Vaccination should also be carried out taking into account all treatment safety requirements and in the presence of means to relieve post-vaccination complications, information about which can be found in the relevant manuals on medical vaccinology.
Literature
- Gusarenko L. A. A case of Lyell's syndrome that developed after an abortive attack of exudative erythema. - Russian Journal of Skin and Venereal Diseases, 1998. - No. 3. — P. 63-67.
- Baykova R. A. Features of the clinic and treatment of exudative erythema multiforme // Dis. Ph.D. - M., 1969.
- Belkin B. G., Sanyan E. Sh. Bulletin of Dermatology, 1973. - No. 6. P. 61.
- Demyanov A.V., Kotov A.Yu., Simbirtsev A.S. Diagnostic value of studying cytokine levels in clinical practice. Cytokines and inflammation. - 2003. - T. 2. - No. 3. — P. 20-35.
- Novikov D.K., Sergeev Yu.V., Novikov P.D. Drug allergy. - M., 2001.
- Novikov D.K., Sergeev Yu.V., Novikov P.D., Sergeev A.Yu. Adverse allergic reactions to drugs and medications in dermatology. Immunopathology, allergology, infectology. - 2003. - No. 3. — P. 45-67.
- Novikov D.K. Medical immunology // Textbook. - Vitebsk, 2002.
- Samgin M. F., Haldin A. A. Herpes simplex. - Moscow, 2002. - P. 160.
- Seagal E. Ya., Banakh V. R., Shirokova T. A. Obstetrics and gynecology, 1975, No. 3. -WITH. 73-74.
- Sokolov E.I., Glan P.V., Grishina T.I., Kuzmenko L.G. et al. Clinical immunology // Guide for doctors. - M.: Medicine, 1998.
- Yarilin A. A. Fundamentals of immunology // Textbook. M.: Medicine. 1999.
- Bushkell et al. J. invest. Derm. vol.74 p.372-374.
- Coltoin A., Mateescu D., Popescu S. et al. Derm-Vener. 1974. v.19, p.99-108.
- Eliaa PM, Fritsh P., Mittermayer HJInvest. Derm.1976, v. 66, p.80-89.
- Epstein E., Flynn P., Davis R., JAMA 1974, v.229, p.425-427.
- Hwang YS, Spruance SL The epidemiologgy of uncommon herpes simplex virus type 1 infections. Herpes Journal, 1999, vol.6(1), p.16-19.
- Leigh Im et al. Clin.exp. Derm.1985, vol. 10, p.58-67.
- Kats J., Livneh A., Shemer J., Danon Y. Herpes-simplex-virus-associated erythema multiforme-a clinical therapeutic dilemma.Pediatr-Dent .1999, 21(6)p.359-362
- Kokuba H., Imafuku S., Burnett J., Aurelian L., Longitudinal study of a patient with herpes-simplex-virus-associated erythema multiforme. Dermatology, 1999,198(3), p. 33-242.
O. L. Ivanov, Doctor of Medical Sciences, Professor M. V. Khaldin MMA named after. I. M. Sechenova, Moscow
The clinical picture is characterized by an acute onset. The disease often begins with prodromal phenomena (fever, malaise, pain in muscles and joints, sore throat).
After the prodromal period, polymorphic rashes on the skin appear in spurts (over 10-15 days or more) - erythema, papules, blisters. Depending on the severity of clinical manifestations, the following forms of the disease are distinguished:
Mild simple (papular) form
The primary morphological elements are hyperemic spots (erythema), papules and vesicles. Papules are round in shape with clear boundaries, ranging in size from 0.3 to 1.5 cm, red-bluish in color, flat, dense on palpation, prone to centrifugal growth with retraction of the central part.
An edematous ridge forms along the periphery of the papules, and the center of the element, gradually sinking, acquires a cyanotic tint (symptom of “target”, or “iris”, or “bull’s eye”. Characteristic are target-shaped lesions less than 3 cm in diameter with clearly defined edges, in structure which are divided into three different zones:
- central disk of dark erythema or purpura that may become necrotic or transform into a dense vesicle
- ring of palpable, pale, swollen area
- outer ring of erythema.
Subjectively, the rash is accompanied by itching. Pathological elements tend to merge to form garlands and arcs. The bubbles are round in shape, small, flat, have a thick covering, are filled with opalescent liquid, and are usually located in the center of the papules.
Secondary morphological elements are erosions, crusts, scales, hyperpigmented spots that do not have clinical features.
The rashes usually appear suddenly, are often located along the periphery, symmetrically on the skin of the dorsum of the feet and hands, palms, extensor surfaces of the forearms and legs, the red border of the lips with the formation of crusts, the mucous membrane of the oral cavity, less often on the neck and torso. Often, only the hands are selectively affected. Damage to the eyes and genitals is observed less frequently. Blisters may form on the mucous membranes, which open to form painful erosions. Resolution of the rash continues within 2-3 weeks, without leaving scars. Pigment spots that appear in place of former papules are yellowish-brown in color.
Vesiculobullous form
The vesiculobullous form occupies an intermediate position between the papular and severe bullous forms. The lesions are represented by erythematous plaques with a bubble in the center and a ring of bubbles along the periphery. Often the mucous membranes are involved in the process.
Severe bullous form
In the severe bullous form (Stevens-Johnson syndrome), the onset is acute, sometimes with prodromal phenomena. Skin, eyes, mucous membranes of the oral cavity, genitals, and perianal area are affected; bronchitis and pneumonia develop. Extensive blisters appear on the oral mucosa with the formation of erosions, which are covered with massive hemorrhagic crusts. The development of catarrhal or purulent conjunctivitis is characteristic, against which blisters may sometimes appear.
Corneal ulceration, uveitis and panophthalmitis with loss of vision often develop. Damage to the mucous membranes of the genitals in men leads to impaired urination with possible involvement of the bladder in the process. Maculopapular rashes or blisters on the skin are noted, less often - pustules, significant in size and number. The period of rash is 2–4 weeks. Skin syndrome is accompanied by fever, pneumonia, nephritis, diarrhea, polyarthritis, etc. Paronychia and otitis media are sometimes associated. Without treatment, the mortality rate for Stevens-Johnson syndrome reaches 5–15%. With all variants of exudative erythema multiforme, there is a tendency to relapse
Publications in the media
Exudative erythema multiforme (erythema multiforme) is an acutely developing disease characterized by the appearance of erythematous spots, bullous lesions of the skin, mucous membranes, and a cyclic relapsing course.
Statistical data . The incidence is 0.3–0.5: 100,000 population, severe forms are observed 2–3 times more often in men.
Classification • Infectious-allergic (idiopathic) form is associated with hyperreactivity to allergens and infectious agents • Toxic-allergic (symptomatic) form is associated with hypersensitivity to drugs • Exudative malignant form (see Stevens-Johnson syndrome) • Rheumatic erythema - round or arcuate lesions erythema on the trunk and limbs, sometimes observed during a rheumatic attack.
Clinical manifestations • Local symptoms •• Symmetrical rashes appear acutely on the skin of the extensor surfaces of the forearms, legs, dorsum of the hands and feet, face, genitals, and mucous membranes. Edema, clearly demarcated, flattened papules of pink-red color, round in shape, with a diameter of several millimeters to 2–5 cm, appear, having two zones: internal (grayish-bluish in color, sometimes with a bubble in the center filled with serous or hemorrhagic contents) and external (red in color [cockade-shaped rashes]) •• Diffuse erythema, blisters, erosive areas covered with a yellowish-gray coating appear on the lips, cheeks, and palate • General symptoms •• Burning and itching in the area of the rash, pain and hyperemia of the mucous membranes, especially mouth and genitals •• Fever •• Headache and joint pain • The most severe manifestation is Stevens-Johnson syndrome • In the toxic-allergic form, unlike the idiopathic one, there is no seasonality of relapses of rashes.
Research methods • Conduct laboratory tests to exclude syphilis - serological reactions, studies for Treponema pallidum • Nikolsky, Asbo-Hansen symptoms are negative, there are no acantholytic cells in the fingerprint smears • During histopathological examination, intra- and intercellular edema, hydropic degeneration of basal cells are noted in the epidermis , in the dermis - swelling of the papillary layer, perivascular infiltrates.
Differential diagnosis • Chicken pox • Bullous pemphigoid • Dühring's dermatitis herpetiformis • Herpes zoster • Syphilitic papular rash.
Treatment • For mild cases - antihistamines and desensitizing agents • For blisters and erosions on the skin - ointments with HA and antibiotics (for example, oxytetracycline + hydrocortisone) • For damage to the oral mucosa - warm rinses with 10% sodium bicarbonate solution, local anesthetics ( 2% lidocaine solution), as well as glucocorticosteroids: dexamethasone (elixir, 0.5 mg per 5 ml of water) 4 times a day followed by swallowing • In more severe cases and with common bullous forms - antibiotics (orally or parenterally), GCs (for example, prednisolone 1–2 mg/kg/day followed by dose reduction), proteolytic enzyme inhibitors (aprotinin). For Stevens-Johnson syndrome - see Stevens-Johnson syndrome.
Forecast . The outcome of the disease in uncomplicated cases is favorable. With Stevens-Johnson syndrome, the mortality rate is 10–30%.
ICD-10 • L51 Erythema multiforme