What role does HPV play in the development of cervical cancer?
Every woman who is sexually active has a risk of being exposed to the HPV virus. Typically, the body's own immune system successfully fights the virus, while other viruses, such as those that cause colds and flu, are spontaneously cleared from the body within a short time. If this does not happen, then HPV can lead to cellular changes that, without timely treatment, can develop into cervical cancer. Women who began sexual activity early and have many sexual partners are especially susceptible to this danger.
The study found that women who have had an HPV infection for 12 to 18 months are up to 300 times more likely to develop cervical cancer than women who are HPV negative. Until now, the so-called PAP smear (PAP) was usually used to prevent cervical cancer.
Who needs to be examined by a doctor?
It is noteworthy that multiple strains of the virus behave differently. HPV may appear on the skin and mucous membranes, or may not manifest itself as long as the carrier’s immunity is normal.
Therefore, we recommend testing for the papilloma virus for people who may fall into one of the following categories:
- sexually active people;
- women and men who have been diagnosed with other sexually transmitted diseases;
- frequently ill patients;
- people of non-traditional sexual orientation;
- pregnant women;
- women with various pathologies in the cervix;
- persons whose partners have been diagnosed with HPV (even if the form is latent, i.e. the virus is present, but inactive at the time of the test).
We recommend getting checked if you have had accidental unprotected sexual intercourse, are in an open relationship, or change partners frequently.
Important! Papillomas on the body can be removed only after histological examination and only with the permission of a doctor. Do not undergo cosmetic procedures or try to remove warts yourself until your HPV type has been determined.
What is a PAP smear?
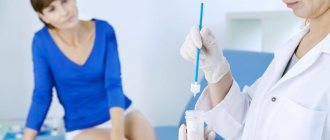
A PAP smear is a test for early detection of cellular changes in the cervix. The test is performed using a small number of cells taken from the cervix and placed on a glass carrier for examination under a microscope. The smear is then examined in a laboratory under a microscope to look for abnormal cells that may indicate the development of the disease.
If abnormal cells are detected, a colposcopy (examination of the cervix using a special device) should be performed to further clarify the diagnosis. But keep this in mind: new research has found that a conventional PAP smear test initially detects about half of all true pre-stage cancers. So the researchers looked for an additional method and found the HPV-Test.
What to do if HPV is detected?
If the test results are disappointing, do not panic. It is necessary to understand how dangerous the detected virus is to health. In most cases, there is no need to take any measures: the immune system of a healthy person can cope with the virus.
If the detected virus is oncogenic, constant monitoring by a doctor is necessary. If a specialist considers the strain dangerous, then constant monitoring and timely action are necessary.
To reduce the risk of contracting the human papillomavirus, use a condom during sexual intercourse. And for testing for HPV, choose medical centers with a good reputation.
You can make an appointment with a specialist in a way convenient for you by calling our contact numbers or using a special form on the website.
What is Daijin Test?
Using the HPV DNA test, the Hybrid Capture® 2 Test (Hybrid Capture 2 HPV DNA-Test) can detect the presence of high-oncogenic risk types of viruses even before cellular changes in the cervix become noticeable. This makes it possible to identify patients at increased risk of the disease much earlier. Don't lose confidence!
A positive result of the “Hybrid Capture 2” HPV DNA test, that is, the detection of viruses, does not mean the presence of pre-stage cancer, or especially cervical cancer itself. This result only indicates a possible risk to your health and gives your doctor the opportunity to monitor you more closely. This way, you can identify the first signs earlier and begin effective treatment. A negative result from the Hybrid Capture 2 HPV DNA test, on the other hand, means that your personal risk of developing cervical cancer is extremely low.
Indications for HPV testing
A person who is infected with HPV may not know about it for a long time. For example, genital warts may appear several weeks after exposure to an infected partner. In this case, the carrier of the virus may not know that he is infected, since he does not have any symptoms.
The incubation period is usually about 3 months, although latent HPV infection can remain in the body for years.
Signs that you should undergo HPV testing:
- the appearance of various formations and growths on the body;
- discharge with an unpleasant odor;
- presence of blood after sexual intercourse;
- discomfort during bowel movements and urination;
- painful sensations during intimacy;
- itching, redness and discomfort in the vaginal and anal areas;
- spotting that is not associated with the menstrual cycle.
An additional indication is to monitor the effectiveness of antiviral therapy.
Testing is also included in screening for precancerous conditions.
Benefits of using the HPV Digene-test®
- Accuracy and reliability: 99%.
- Sensitivity: over 95%.
- The specificity of the test is equivalent to cytological examination.
- Ease of use, standardization and objectivity.
The Digene-test® HPV technology is ideal for routine diagnostics:
- The research is automated, provided with automatic verification of results and issuance of conclusions.
- The research time is record-breakingly short (2.5 hours), which significantly increases the profitability of the method.
- The use of a stripped 96-well microplate format allows for testing with any number of samples and increases productivity.
- The collected cervical DNA sample can be further tested for chlamydia and gonorrhea.
HPV technology Digene-test®
The HPV Digene-test® technology consists of a unique method of binding viral DNA to an RNA probe, capturing the resulting hybrid with monoclonal antibodies and chemiluminescent detection of the resulting complexes. That is why the Digene HPV test is also called the Digene Hybrid Capture® method.
The procedure is carried out in 5 simple steps.
- Cell lysis and DNA denaturation directly in the cervical canal collection tube.
- Hybridization is the formation of a hybrid between viral DNA and RNA probe.
- Capture of the resulting hybrid with monoclonal antibodies on the solid phase.
- Binding of the captured hybrid by enzyme-labeled antibodies.
- Chemiluminescence measurement.
No other type of cancer can be prevented as effectively by cervical cancer screening as cervical cancer. The introduction of cervical cytology screening and treatment of higher grade dysplasia has significantly reduced the incidence, morbidity and mortality of cervical cancer.
Prerequisites and structure of screening using the example of Switzerland
In Switzerland, cervical cancer screening was introduced in the late 1960s by gynecologists and family doctors. Since the introduction of screening, the incidence has decreased by more than 60%. The incidence of cervical cancer in Switzerland is one of the lowest in the world (4.0/100,000 inhabitants).
In Switzerland, there is opportunistic screening, which requires women to make their own appointment with their doctor, and the doctor is responsible for ensuring that the screening is carried out correctly and that if abnormal features are detected, appropriate action is taken.
In contrast, many countries have organized screening programs whereby authorities invite women to be examined by their GP and abnormal results are subsequently monitored. Central data management allows statistical evaluation. In Switzerland, it is difficult to obtain reliable data on the incidence and prevalence of dysplasia. The only data come from observational studies from the Federal Health Administration and the National Institute of Cancer Epidemiology. These data suggest that approximately 30% of all eligible women never participate in cervical cancer screening, that women with low levels of education and women living in rural areas are at higher risk of cervical cancer, and that 50% of all women who develop cervical cancer have had normal screening results within the past 3–5 years.
It is necessary to evaluate all the positive and negative features of cancer screening. The desire to achieve the highest possible detection rate with a particular method must be weighed against its potential disadvantages. Disadvantages of screening include psychological distress, unnecessary treatment of precancer, complications from dysplasia treatment in subsequent pregnancies, and associated health care costs.
Screening for each age phase:
Start of cancer screening at age 21
In most countries with an organized cancer screening program, women begin to be screened after they reach the age of 25 years.
Women under 21 years of age should not be screened for cervical cancer, regardless of sexual debut or other risk factors. The incidence of cervical cancer in this age group is very low, and there is no evidence that the benefits of screening outweigh the harms in this age group. We recommend cancer screening for women aged 21 years in the absence of an organized cancer screening program. If higher rates of HPV vaccination are achieved in the future, initiation of cancer screening at older ages will need to be re-discussed.
Stopping cancer screening at age 70
There have been no randomized controlled trials that demonstrate the benefit of cancer screening in patients over 65 years of age, either based on HPV (human papillomavirus) testing or cytology. Individual surveillance studies suggest that cancer screening in women over 65 years of age may be appropriate.
Screening may be discontinued at age 70 if:
- 3 cytology tests performed within the last 10 years were normal or 2 HPV tests performed within the last 3 years were negative;
- The patient has never had an anogenital lesion associated with high-grade HPV. If there is a history of such a lesion, cancer screening should be carried out even after 70 years.
Method and interval of cancer screening in women aged 20 to 30 years
Cytological screening every 3 years
In women under 30 years of age, cancer screening should be based on cytological examination, since the prevalence of HPV in this age group is very high. For the same reason, cotesting (cytological examination plus HPV analysis) should not be performed. A 2012 expert report recommended cancer screening in this age group every 2 years. This interval is not consistent with any scientific evidence and may cause more harm than good in terms of psychological distress, unnecessary additional testing and interventions with adverse consequences for subsequent pregnancies.
Therefore, according to the new recommendations, cytological screening in women aged 21 to 29 years is recommended to be carried out once every 3 years.
Method and interval of cancer screening in women aged 30 to 70 years
Below are both possible methods of primary cancer screening (cytological examination or HPV analysis).
Cytological screening every 3 years
The interval between screening studies of 3 years in patients aged 30 to 70 years also demonstrates the most optimal balance between benefit and harm. A longer interval cannot be established based on clinical trial data, nor can an interval of less than once every 3 years, as this may lead to possible overtreatment of transitional dysplasia and be associated with negative consequences such as psychological stress, vaginal bleeding, infections and adverse effects. during pregnancy.
HPV test once every 3 years
The development of tests for diagnosing HPV has led to important changes in our methods of screening for cervical cancer. The vast majority of meta-analysis data from randomized controlled trials as well as long-term cohort studies indicate that HPV screening is more sensitive in detecting CIN2+ histologic dysplasia and progressive glandular dysplasia than cytologic screening. Among all cases of cervical cancer, adenocarcinomas account for the highest proportion. The risk of developing CIN2+ dysplasia after a negative high-risk HPV test over the next 5 years is very low. For example, in 4 European countries, HPV screening provides 60-70% greater protection against cervical cancer than cytology. The negative predictive value (NPT) is high, allowing for a safe and cost-effective extension of the cervical cancer screening interval. Current international guidelines for HPV-based screening recommend that HPV testing be performed as part of an organized screening program every 5 to 10 years.
Compliance with follow-up testing when HPV is detected is very important. Especially when HPV16/18 is detected, there is an increased risk of developing CIN2+ dysplasia (approximately 20% in the next 10 years).
Cotesting (simultaneous cytological examination and HPV testing) is not recommended
In 2012, the United States became the first country to introduce opportunistic screening as a cotest for women aged 30 to 65 years, administered once every 5 years. An evaluation of this screening method showed that the sensitivity of cotesting is only slightly higher than the sensitivity of HPV-only screening, but its specificity is significantly lower. This leads to a threefold increase in the number of cases of colposcopy to further clarify the diagnosis. For this reason, it is recommended that cotesting be abandoned in favor of HPV testing alone, as the additional benefit of cytology has not been demonstrated. There is no evidence that cotesting performed every 5 years is superior to HPV-only testing performed every 3 years.
Cytological examination or screening for HPV?
The HPV test has much higher sensitivity. This contrasts with the slightly better specificity of cytology and the higher cost of HPV testing.
Cytology or HPV screening after hysterectomy?
There have been no randomized controlled trials that have examined both screening methods. After removal of the border between the squamous and columnar epithelium through a total hysterectomy, cervical cancer should not be expected to occur. Because vaginal carcinomas are very rare, women with a history of no abnormal cervical cytology/histology prior to hysterectomy should not undergo further screening. In women with post-cervical dysplasia, or in whom high-risk HPV has been identified, continued screening is recommended. Women with the condition following supracervical hysterectomy are advised to continue screening at the same interval.
Liquid Based Cytology (LBC) or conventional cytology?
At present, there is still no evidence that LBC is more effective than conventional cytology.
The advantage of LBC is that HPV testing can be done using the same material or, in the case of a positive HPV test, reflex cytology can be performed without the need to return the patient for a repeat procedure.
Cytological results should be assessed according to the Bethesda classification.
Screening in the absence of endocervical cells in the material for cytological examination
According to Bethesda nomenclature, cervical cytology results can be reasonably interpreted even in the absence of endocervical cells. In this case, if normal results are obtained III, a repeat examination is recommended after a year.
Screening after conization
In the period after treatment of cervical intraepithelial neoplasia, cotesting (HPV test + cytological examination) should be performed. It should be carried out 6, 12 and 24 months after therapy. Cotesting makes it possible to predict recurrence of dysplasia much more effectively than cytological examination alone. If cotesting results are negative after conization, there is a 1% risk of developing CIN2+ over the next 5 years and a 3.6% risk of developing CIN3+ dysplasia over the next 10 years (0% over 5 and 10 years). If normal results are obtained, the patient should subsequently undergo routine preventive medical examinations.
If pathological features are detected (= at least 1 stage of the test gave positive results), differential colposcopy should be performed.
In squamous cell dysplasia, a “resection margin with a positive reaction” (R1) on histological examination of a cervical cone preparation has low sensitivity for the persistence of CIN after treatment of CIN-2/3 dysplasia and is not an indication for immediate re-conization. Negative HPV test results after CIN therapy exclude persistent CIN or recurrence of CIN even in the condition after incomplete resection.
In case of incomplete resection of adenocarcinoma in situ (AIS), repeat conization or, if the patient does not desire to have children, hysterectomy is recommended.
Screening in women after HPV vaccination
Currently, women who have not and have received HPV vaccination should be screened by cytology or HPV testing, as research evidence is still insufficient to change the interval.
What HPV test can be used for initial HPV screening?
In order to perform primary screening for cervical cancer using an HPV test, the test must be validated. To do this, he must meet the Meyer criteria (see table of currently approved HPV tests).
The name of the analysis performed must be indicated along with the result of the analysis.
The following HPV tests are encouraged for use:
- Cobas Taqman 4800 HPV (Roche Diagnostics)
- Abbott RT High-risk HPV Test
- APTIMA HPV Assay (Hologic)
- Seegene Anyplex II HR
- Cervista™ HPV HR and Genfind™ DNA Extraction Kit (Hologic)
- Digene Hybrid Capture 2 High-Risk HPV DNA Test (QIAGEN Gaithersburg, Inc.)
- BD Onclarity HPV Test
- Papillocheck HPV Test
- Cepheid Xpert HPV.
In-house HPV tests may be used as a second option (eg for genotyping as a follow-up test after abnormal cytology/histology results) if quality control has been performed according to international standards. Ideally, the results of studies using these assays should be published or the assays should be based on published research methods. However, such HPV tests should never be used as first-line tests or for initial HPV screening.
Screening in immunocompromised women
Patients with immunosuppression (regardless of cause) have a significantly higher risk of HPV-related dysplasia and should be monitored by an experienced colposcopist.
Contact us
We are always ready to advise you on screening for cervical cancer or any other diseases in the field of gynecology. Write or call us and we will always help you: Email: [email protected] Tel.: +49 212 5476913 Viber | WhatsApp: +49 173-2034066 | +49 177-5404270 For your convenience, please save the phone number in your phone book and call or write to us for free on WhatsApp, Viber or Telegram. Applications made on weekends or holidays will be processed on the first business day. In urgent cases, request processing is carried out on weekends and holidays.
Deciphering the analysis for human papillomavirus
The future treatment will depend on the result of the human papillomavirus test.
The result is deciphered as “detected” or “not detected”. If fragments of the DNA chain of papillomavirus type 16 or 18 were found in the sample for analysis, this indicates the fact of infection with this virus. If the test response for papilloma virus is “not detected,” it means that either the concentration of viral material was below the sensitivity limit of the method, or viral DNA is completely absent from the sample.
It has already been mentioned that with human papillomavirus, a photo or visual examination usually cannot provide information about the risk of a malignant process or its onset. Therefore, tests that allow not only to establish the fact of infection, but also to give an objective prognosis on the risk of developing a malignant process in each specific case are of great importance.
Digene-Test is another modern diagnostic method that allows not only to determine the fact of infection with the papilloma virus and whether it is an oncogenic or non-oncogenic type, but also provides information that the virus has reached a concentration dangerous for the development of a tumor. If this test detects a dangerous concentration of oncogenic human papillomavirus, treatment that is started as early as possible can save you from developing a tumor.