Herpesviruses 6, 7, 8 types
Over the past 15 years, three new anthroponotic representatives of this family have been identified - HHV-6, HHV-7, HHV-8, which are characterized by a variety of clinical manifestations and asymptomatic carriage.
HHV-6
Herpesvirus type 6 (HHV-6, HHV-6) is relatively recently included in the list of known human pathogens and is a serious contender for the role of the etiological agent of multiple sclerosis and fever of newborns with convulsive syndrome, infectious mononucleosis negative for the Epstein-Barr virus (EBV) and cytomegalovirus (CMV) and HHV-6-associated encephalitis. HHV-6 is also a cofactor for AIDS, some forms of cervical carcinomas, and nasopharyngeal carcinomas.
HHV-6 was first isolated in 1986 from peripheral blood lymphocytes of patients with various lymphoproliferative diseases, including those infected with HIV. The new virus was named human B-lymphotropic virus (HBLV - human B-lymphotropic virus). HBLV infected only fresh B cells in vitro. Later, a wider spectrum of cellular tropism, predominantly towards T cells, was described, as a result of which the virus was renamed HHV-6.
HHV-6 is similar to other herpes viruses, but differs from them in biological, immunological properties, spectrum of sensitive cells, antigenic structure, genome composition, number and molecular weight of structural viral proteins. The virion diameter is 160–200 nm, the type of symmetry is icosahedral, contains 162 capsomeres, and has a supercapsid lipid-containing shell. The genome is represented by double-stranded DNA. Restriction analysis of HHV-6 DNA revealed genome variability among different virus isolates. When comparing the primary structure of the genomes of HHV-6 and CMV, a certain similarity was discovered. The degree of homology between HHV-6 and CMV was greater than between HHV-6 and other herpesviruses, indicating that the genomes of these two viruses are closely related.
Studies of HHV-6 isolates from people with various pathologies have shown that they belong to two variants: A or B (HHV-6A and HHV-6B). Variants differ in in vitro cellular tropism, restriction endonuclease profile, nucleotide sequence, reactivity with monoclonal antibodies, seroepidemiology and involvement in various diseases. Infection induced by HHV-6A is observed less frequently, and the role of this variant of the virus in human pathology is not sufficiently clear, and HHV-6B is the main etiopathogen of sudden exanthema (Exantema subitum).
In vitro replication. The HHV-6 virus is selectively tropic for CD4+ T cells, but is also capable of infecting T cells with CD3+, CD5+, CD7+, CD8– determinants. The virus replicates in many primary and continuous cell cultures of various origins: T-series lymphocytes, monocyte-macrophage, megakaryocytes, glial cells, thymus cells, and freshly isolated human lymphocytes. The growth cycle of the virus lasts 4–5 days. The enveloped virion was identified by electron microscopy on the 5th day after infection in the cytoplasm of cells and extracellularly; viral DNA and nucleocapsid - on the 3rd day. On day 5, HHV-6-infected cells formed syncytia with nuclear and cytoplasmic inclusions, large cells, and virus reproduction was accompanied by cell destruction and lysis. On days 5–10, almost 90% of all cells were infected with the virus.
Epidemiology of HHV-6 infection. The first clinical and epidemiological studies conducted in 1986 revealed the presence of this infection in a recreation area in Nevada (USA). Clinically, the disease was expressed in flu-like symptoms with fever, night sweats, swollen lymph nodes, and a number of psychological symptoms (fatigue, depression). The disease is called “chronic fatigue syndrome.” At the same time, antibodies to HHV-6 were detected in 75% of patients. A year later, the first patient with a clinically similar disease and antibodies to HHV-6 in the blood was registered in Germany. Subsequently, this infection was identified in Europe (England, Sweden) and Africa. However, various researchers have reported the isolation of HHV-6 from blood cells not only from individuals with various lymphoproliferative and hematological diseases, infected with HTLV-1, HIV-1 and HIV-2 or patients with AIDS, but also from healthy adults.
According to Russian authors, 80% of healthy donors, 65% of HIV-infected and 73% of cancer patients have antibodies to HHV-6. At birth, most children are seropositive due to maternal antibodies, the titer of which decreases by 5 months. However, by one year, the percentage of seropositive babies is the same as among older children and adults. The high frequency of antibody detection and the early age of infection indicate the presence of the virus in the immediate environment. Isolation of HHV-6, detection of viral proteins and DNA in saliva and sputum samples indicate that the virus resides in the salivary glands in humans, and in vitro experiments have shown that it persists in a latent phase in monocytes/macrophages. Under natural conditions, the main route of transmission of the virus is airborne droplets. A vertical route of infection cannot be ruled out; Viral antigens were present in abortive material in a number of cases of spontaneous abortions. Sexual transmission of the virus and perinatal infection cannot be ruled out either. Long-term reproduction during acute infection and persistence of HHV-6 in the blood cells of apparently healthy people, including donors, is a serious risk factor for transmission of the virus during transfusion of blood and its components, organ and tissue transplantation. Post-transplant serological conversion to HHV-6 is observed in 43% of recipients. At the same time, the increased titer of antibodies to HHV-6 in the blood of recipients persisted for 2 years after transplantation.
Diagnostics. Diagnosis of HHV-6 infection is based on a comparative analysis of the results of virus isolation on sensitive cell cultures from a patient, detection of viral proteins (using immunoperoxidase and immunofluorescence methods, electron microscopy) or its genome (using hybridization and polymerase chain reaction (PCR) methods) in materials from the patient and detection of virus-specific antibodies in the blood. Virus-specific immunoglobulins of class M (IgM) are synthesized 2 weeks after acute primary infection, reaching a titer of 1:1280, according to indirect immunofluorescence, and class G (IgG) - a week later and reach a higher titer of 1:10240. Since the IgM titer decreases rapidly and disappears after 4–6 weeks, acute primary HHV-6 infection is diagnosed by the presence of IgG (a titer greater than 1:20 is considered positive). IgM synthesis is possible both during primary and during reactivation of latent HHV-6 infection. Periodic reactivation and active persistent HHV-6 infection in patients with a defective immune response, as with other herpesvirus infections, leads to an increase in the titer of virus-specific IgG, often without a corresponding increase in the titer of IgM.
Primary HHV-6 infection in immunocompetent individuals is diagnosed based on the correlation of the presence and increase in titers of virus-specific IgG and IgM with the dynamics of the development of clinical manifestations of the disease. Serological testing must be complemented by virus isolation and/or genome detection. With immune disorders and reactivation of HHV-6 infection, the number of such genome-bearing cells increases to 50–70, and the detection of genome-bearing cells in the range of 200–7500 indicates a pathogenetic connection of the reactivated HHV-6 infection with the disease.
Clinic. The range of diseases associated with HHV-6 is quite wide. The polymorphism of the pathology depends both on the form of infection and on strain differences of the virus.
Diseases associated with primary acute HHV-6 infection include: chronic fatigue syndrome (myalgic encephalomyelitis); sudden exanthema in newborns and older children (roseola infantum exantema subitum); infectious mononucleosis in adolescents and adults not associated with EBV infection; histiocytic necrotizing lymphadenitis (KiKuchi's lymphadenitis).
Diseases associated with persistent HHV-6 infection include: lymphoproliferative diseases (immunodeficiency, lymphadenopathy, polyclonal lymphoproliferation); malignant lymphomas (non-Hodgkin's lymphoma, peripheral T-cell leukemia, B-cell lymphoma, dermatopathic lymphadenopathy, Hodgkin's disease, sinusoidal B-cell lymphoma, pleomorphic T-cell lymphoma).
HHV-6 is associated with various lymphoproliferative and immunosuppressive diseases, sudden exanthema of newborns, malignant neoplasms, autoimmune pathology, some diseases of the central nervous system, etc. Reinfection with HHV-6 is observed in patients with impaired immune status, immunosuppression (organ transplantation, AIDS, etc.) .
The etiological role of HHV-6 in the development of sudden exanthema, a widespread disease in children aged 3 months to 3 years, has been proven. In some cases, the cause of fulminant exanthema is HHV-7. The incubation period of the disease is 5–15 days. The disease is characterized by high fever (38.5–40°C) for 3–5 days, followed by an erythematous or macular skin rash for 1–3 days, which coincides with a decrease in temperature. About half of all cases of first-ever neonatal fever are associated with primary infection with HHV-6.
The HHV-6B variant is predominantly associated with the development of exanthema. During the period of fever (and even before that), the virus is present in CD4+ cells; at this time, it is possible to isolate the virus from the blood of patients in more than 90% of cases.
Antibodies to HHV-6 are absent in the acute phase of the disease and begin to be detected in most cases on the 6-8th day from the onset of the disease. IgM antibodies are released on the 5th day of fever and are detected within 2–3 weeks. IgG antibodies appear on the 7th day, reaching a maximum after 2–3 weeks, and persist for a long time. Usually the disease ends without complications, but clinical cases of manifest infection with various symptoms have been described: fever above 40°C, inflammation of the eardrum, respiratory and gastrointestinal symptoms, neurological complications (encephalitis, meningoencephalitis, aseptic meningitis, seizures followed by epilepsy); There are reports of primary HHV-6 infection with hepatosplenomegaly, fatal fulminant hepatitis, and fatal disseminated infection.
The potential tumorigenicity of HHV-6 and its role in the multistep process of tumorigenesis is supported by the following facts. HHV-6 activates human papillomavirus E6 and E7 oncoproteins in cervical carcinoma. HHV-6 DNA is capable of inducing neoplastic transformation of NIH3T3 cells in vitro and in animal models.
When analyzing saliva samples from patients with nasopharyngeal carcinoma, the PCR method revealed HHV-6 DNA in a number of cases. In these same patients, a significant increase in titers of antibodies to HHV-6 of the IgG class was noted and, in contrast to the healthy population, antibodies of the IgA class were found. Thus, it can be assumed that the presence of IgA antibodies to HHV-6 may serve as a sign of nasopharyngeal carcinoma.
Using PCR, in situ hybridization, HHV-6 DNA was identified in tissues and cells of biopsy samples of Hodgkin's, mixed B- and T-cell non-Hodgkin's lymphomas, angioimmunoblastoid lymphadenopathy, African Burkitt's lymphoma, T-cell acute lymphoblastoid leukemia, lymphogranulomatosis, infectious mononucleosis , not associated with EBV, and a number of other lymphoproliferative diseases.
The role of HHV-6 in chronic fatigue syndrome has been debated by various authors, but the evidence provided to support this hypothesis is mixed.
HHV-7
HHV-7 was isolated for the first time from CD+ T cells from healthy individuals. DNA hybridization analysis showed that HHV-7 is different from already known herpes viruses, but has identical regions with CMV and HHV-6.
The history of the discovery of this virus is as follows: in 1990, while studying HIV-1 at the US Military Medical Research Institute, it was noted that in 13-day cultures of uninfected activated CD4+ T cells obtained from an apparently healthy 26-year-old subject , a spontaneous cytopathic effect develops, characterized by “balloonization” of cells and a small syncytium. At the same time, the cells were free from HIV infection, which was confirmed by the absence of HIV p24 and reverse transcriptase activity. These cell cultures were transferred to the National Institute of Allergy and Infectious Diseases (USA), where, in the course of further research, the above isolate was designated as HHV-7 - HHV-7.
Later, a virus with similar characteristics was isolated by other researchers.
Seroepidemiological studies have shown that HHV-7 is widespread. The isolation rate of HHV-7 in children aged 0-11 months is 0%, 12-23 months - 50%, 24-35 months - 75%, over 36 months - 100% (unlike HHV-6, in which seroconversion observed before the age of 12 months). In the blood of donors, HHV-7 DNA was detected in 97.3% of the examined individuals, while carriage of the HHV-7 genome was observed for 53 weeks. Thus, HHV-7 persists in the host after the primary infection and is most often isolated from healthy adults.
Electron microscopic examination of ultrathin sections of cells infected with HHV-7 revealed virions with a diameter of up to 170 nm and DNA of 150 kb, typical for a herpes virus. The virions contained an electron-dense cylindrical core, capsid, tegument, and outer envelope and had significant morphological similarity to HHV-6. Hybridization analysis showed that HHV-7 DNA is different from that of herpes simplex virus, EBV, herpes zoster, and CMV. At the same time, CMV DNA has limited cross-hybridization with HHV-7 DNA (homology is at the level of 36%). Slightly greater similarity was found between HHV DNA and HHV-7 DNA (according to various sources, homology is at the level of 57.5–58.8%). However, based on a number of criteria, we can conclude that we are talking about different viruses, and not about different strains of the same virus. To date, HHV-7 has been classified as a member of the β-herpesvirus subfamily.
The prevalence of infection and routes of transmission are unknown. There is also evidence of the isolation of HHV-7 from the saliva of infected people, as well as the persistence of the virus in T-lymphocytes, which suggests the possibility of airborne transmission of infection, especially in young children, and transmission of infection through transfusion of blood and its components . To isolate and identify HHV-7, peripheral blood mononuclear cell culture, indirect immunofluorescence, electron microscopy, molecular hybridization and DNA sequence analysis are used.
The significance of HHV-7 in pathology has not been studied. HHV-7 is associated with lymphoproliferative diseases, chronic fatigue syndromes, and immunodeficiency syndromes.
Chronic fatigue syndrome was first identified as an independent disease in 1988 by the Centers for Disease Control–CDC, Atlanta, USA. The reason for this was a sudden increase in the state of Nevada in 1984 in the number of patients complaining of constant fatigue, accompanied by a number of somatic and psychological symptoms - in the absence of a visible cause of the disease. Similar outbreaks of the disease were observed before: in Los Angeles in 1934, in Iceland in 1948, in London in 1955, in Florida in 1956. Work on the study of chronic fatigue syndrome in the USA was led by P. Cheney and D. Peterson Soon, special clinics for these patients were opened in the United States and a National Association was created. This disease is currently being studied in the UK, Germany, Australia, Japan and other countries.
A report published by the CDC in March 1988 in the Annals of Internal Medicine established diagnostic criteria (major and minor) for chronic fatigue syndrome. This diagnostic complex was revised in 1991, 1992, 1994. Major (mandatory) diagnostic criteria include constant fatigue and a decrease in performance by 50% or more observed for at least 6 months in previously healthy people. The second mandatory criterion is the absence of diseases or other causes that can cause such a condition.
Small criteria can be combined into several groups. The first of them includes symptoms reflecting the presence of a chronic infectious process: low-grade fever, chronic pharyngitis, enlarged lymph nodes (cervical, occipital, axillary), muscle and joint pain. The second group is mental and psychological problems: sleep disturbance (hypo- or hypersomnia), memory loss, increased irritability, decreased intelligence, inability to concentrate, depression, etc.). The third group consisted of symptoms of autonomic-endocrine dysfunction: rapid change in body weight, dysfunction of the gastrointestinal tract, decreased appetite, arrhythmia, dysuria, rapid physical fatigue followed by prolonged (more than 24 hours) fatigue, etc. The fourth group combines symptoms of allergies and increased sensitivity to medications, insolation, alcohol and some other factors.
According to the 1994 diagnostic criteria, the diagnosis of chronic fatigue syndrome is considered reliable if the patient has two mandatory criteria and four of the following eight additional signs (which have also been observed for at least 6 months): impaired memory or concentration; pharyngitis; painful cervical lymph nodes; muscle pain; polyarthralgia; unusual, new headache for the patient; unrefreshing sleep; malaise after physical exertion.
The prevalence of chronic fatigue syndrome, according to most researchers, is approximately the same in different countries and socio-demographic groups. People of any age are susceptible to the disease, but it has been noted that women aged 25–49 years are affected more often than men.
It is assumed that HHV-7 may be the cause of exanthema subitum, but not directly, but indirectly, due to the reactivation of HHV-6 from a latent state. HHV-7 is selectively tropic for CD+ T cells. The virus has been shown to infect CD4+ in 42% and CD8+ in 4% of cases. When HHV-7 and HIV interact, a competing effect appears for the order of infection of CD+ lymphocytes.
HHV-8
HHV-8 is a herpesvirus associated with Kaposi's sarcoma (KS). Was recently discovered and identified by molecular cloning using Kaposi's sarcoma tissue. KS is a multifocal malignant tumor of vascular origin with predominant lesions of the skin and involvement of the internal organs and lymph nodes. There are four different epidemiological forms of KS: classical, African, iatrogenic and AIDS-associated KS. HHV-8 is divided into three variants: A, B and C, based on differences in the nucleotide sequences of genome subsegments.
Option A is associated with classic KS and with AIDS-associated lesions of the skin and internal organs, and B and C are associated with lymphoproliferative diseases (lymphomas, generalized lymphadenopathy, Castleman's disease).
The viral theory of the development of KS was first put forward in 1967 by B. MacKinney, who studied its epidemiology in African residents. Subsequently, this concept was supported by many other scientists who suggested the involvement of Herpes group viruses in this disease: EBV, CMV in association with herpes simplex virus immunotypes 1 and 2, and human T-cell lymphoma virus (HTLV).
A new stage in understanding the role of viruses in the development of KS came in 1994, when Y. Chang and co-authors discovered in PCR using the primer KS 330233 they synthesized the presence of a unique DNA sequence in the genome of tumor cells isolated from a biopsy of skin lesions of a patient with AIDS -associated type KS.
Taking into account the high frequency of detection of HHV-8 in the cells of KS lesions, data from epidemiological studies (detection of HHV-8 in lesions of patients with various types of KS from both endemic and non-endemic regions), as well as confirmation of the transforming potential of some of its genes, this virus is currently considered as the most likely candidate for the role of the etiological factor of this disease. This is confirmed by the biological properties of HHV-8: it was found that it encodes proteins that control cell growth and proliferation, and has genetic affinity for representatives of the rhadinovirus subfamily, which have transforming properties. The etiological role of HHV-8 in the development of KS has also been confirmed in Russia. Moreover, serum antibodies to this virus were detected in healthy donors from Russia in 9.6% of cases.
The data confirm the possibility of virus persistence in groups of healthy individuals and its activation under conditions of immunosuppression.
The high frequency of detection of HHV-8 in the ejaculate of healthy donors from Italy, which is also a region endemic for KS, does not exclude the possibility of sexual transmission of this infection. In this regard, the data of S. Lin and co-authors (1995) are of interest, showing that 13 out of 30 HIV-positive patients with the presence of HHV-8 in the ejaculate developed KS within 5 years, while in the ejaculate of 30 HIV-negative patients without HHV-8 during the same observation period, KS did not develop in any case. The studies carried out by domestic authors and the data they obtained indicate that the tissues of the urogenital tract in some cases can be the site of HHV-8 latency. At the same time, the high frequency of chronic inflammatory lesions of the genitourinary organs in the group of patients with KS should be considered as a factor that disrupts the blood-testis barrier and thereby facilitates the penetration of inflammatory cells into the ejaculate, including HHV-8-infected B lymphocytes. It is necessary to take into account the role of chronic inflammation in reducing general immunity and local immune control in the tissues of the reproductive system. Such inflammation can lead to the activation of HHV-8 that persists there. It has been established that up to 10% of HHV-8 viruses detected in KS lesions are in the lytic phase, and therefore this virus should be detected with high frequency in peripheral blood lymphocytes. However, HHV-8 in peripheral blood lymphocytes is detected, as a rule, only in patients with severe immunosuppression (AIDS-associated and immunosuppressive type of disease), while in patients with other types of KS, infected cells are contained in lesions in the ejaculate, and from the blood, Apparently, they are eliminated quite effectively. HHV-8 was detected in the ejaculate only if patients with KS had a chronic inflammatory process of the genitourinary organs (chronic urethroprostatitis). Thus, ejaculate from patients with KS is a natural reservoir for HHV-8 infection. Lymphocytes infected with the HHV-8 virus penetrate into the ejaculate and remain in it under conditions of accumulation of CD8+ T-lymphocytes, which inhibit the immune response not only to sperm, but also to these infected cells.
The virus is widespread in the population: more than 25% of the adult population and 90% of HIV-infected people have antibodies to the lytic proteins of HHV-8. HHV-8, like EBV or HVS, primarily infects lymphocytes and is associated with cellular transformation and immortalization. HHV-8 is a possible etiological agent of all forms of KS and is associated with the development of some B-cell lymphomas, angioimmunoblastoid lymphadenopathy, Castleman disease and a number of other lymphoproliferative diseases.
Despite the large arsenal of antiherpetic drugs, treatment of diseases caused by these viruses presents significant difficulties. This is due to the genotypic characteristics of the pathogen, long-term persistence of the virus in the body, and varying sensitivity to drugs. Studies of the antiviral effect of some drugs have shown that HHV-6, 7, 8 are insensitive to nucleoside analogues. Ganciclovir and foscarnet have been used in treatment with some success. However, drugs that would be sufficiently effective in treating infection caused by HHV-6, 7, 8 have not yet been found.
Literature
- Isakov V. A., Borisova V. V., Isakov D. V. Herpes: pathogenesis and laboratory diagnosis: A guide for doctors. St. Petersburg: Lan, 1999.
- Isakov V. A. Modern methods of treating herpetic infection // TERRA MEDICA NOVA. St. Petersburg, 1997. No. 3. P. 2-7.
- Bekhalo V. A., Lovenetsky A. N. Clinic, treatment and laboratory diagnosis of human herpesvirus diseases: A guide for doctors. M.: Nearmedic plus, 1998.
- Ershov F.I., Ospelnikova T.P. Modern arsenal of antiherpetic drugs // Infections and antimicrobial therapy. M.: Media Medica, 2001. T. 3. No. 4. P. 100-104.
- Panchenko L. A., Kirichenko I. I., Khodak L. A. Causative agents of herpes virus infections and the most important clinical manifestations in humans // Pharmacist. 1999.
- Murzich A.V., Golubev M.A. Herpetic infection//South Russian Medical Journal. 1998. No. 3.
- Khakhalin L.N., Solovyova E.V. Human herpesvirus diseases//Clinical pharmacology and therapy. 1998. T. 7.
- Korneev A.V., Artsimovich N.G. Chronic fatigue syndrome and immune dysfunction // Attending Physician. 1998. No. 3.
- Malashenkova I.K., Didkovsky N.A. Chronic fatigue syndrome//Breast cancer. 1997. T. 5. No. 12.
- Lvov N. D., Melnichenko A. V. Human herpes viruses types 6, 7 and 8 are new pathogens of the Herpesviridae family // Questions of Virology. 1999. T. 44. No. 3.
- Kolomiets A.G., Kolomiets N.D. New human herpesviruses and the pathology they cause // Clinical Medicine. 1997. No. 1.
- Perminova N. G., Timofeev I. V., Paletskaya T. F., Maksyutov A. Z., Kozhina E. M. Herpes virus type 6 (HHV-6): current state of the issue // Bulletin of the Russian Academy of Medical Sciences. 1998. No. 4. pp. 21-24.
- Molochkov A. V., Kadyrova E. L., Kartashova M. G., Budoragin E. S., Gurtsevich V. E., Molochkov V. A. On the detection of human herpes virus type 8 in the tissues of the urogenital tract in idiopathic Kaposi's sarcoma // Ross. magazine skin and Venus. diseases. 2001. No. 6. P. 7-10.
- Barinsky I.F., Shubladze A.K., Kasparov A.A., Grebenyuk V.N. Herpes: etiology, diagnosis, treatment. M.: Medicine, 1986.
E. G. Belova, Candidate of Medical Sciences, Associate Professor T. K. Kuskova, Candidate of Medical Sciences MGMSU, Moscow
Symptoms
As a rule, symptoms appear only in young children or in adults with a pathology of the immune system, HIV-infected people taking medications that suppress the immune system. It can occur after serious illnesses, transplantation and other surgical interventions. Symptoms are quite extensive, but are more often expressed in fever and rash on the skin and mucous membranes.
Cytomegalovirus infection
- In the acquired form, the following is observed:
- Temperature increase.
- General malaise, weakness.
- Headache.
- Enlarged neck lymph nodes, sore throat.
Rarely, complications occur in the form of pneumonia, arthritis, and symptoms of hepatitis.
Congenital is expressed in the following symptoms:
- Hepatosplenomegaly is an enlargement of the spleen and liver.
- Microcephaly is a significant reduction in the size of the skull and, accordingly, the brain.
- Jaundice of the skin and sclera.
- Petechiae is a hemorrhagic rash that occurs due to damage to intradermal capillaries.
- Neurological abnormalities.
Infection with cytomegalovirus during pregnancy can lead to the death of the embryo and pathological damage to the fetus. These include hemorrhagic syndrome and damage to the nervous system. Sensorineural hearing loss and balance disorders are more common, and intellectual disorders, epilepsy, cerebral palsy, visual impairment, and microcephaly are less common.
Epstein–Barr virus
Primary infection is characterized by:
- Catarrhal sore throat.
- Nasal congestion.
- Enlarged lymph nodes.
- Moderate increase in lymphocytes.
Manifest form - infectious mononucleosis is accompanied by:
- fever;
- general malaise;
- headache;
- sore throat;
- enlarged cervical lymph nodes, pain;
- splenomegaly;
- hepatomegaly;
- jaundice.
Infectious mononucleosis:
- Heat.
- Headache.
- Inflammation of the pharynx and tonsils.
- Enlarged and painful lymph nodes.
- Enlarged liver and spleen.
In severe forms of the disease, complications may occur in the form of otitis media, sinusitis, hepatitis, peritonsillar abscess, and pneumonia.
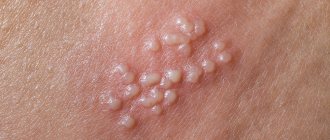
Herpes virus HHV-6
It has a wide range of symptoms, with the following symptoms most often noted:
- temperature up to +40 °C;
- symptoms of intoxication;
- erythematous or papular rash;
- mild hyperemia of the pharynx.
If a person has a strong immune system, without pathologies, then HCH-6 is asymptomatic.
Herpes virus HHV-7
No symptoms identified. In persons with immunodeficiency, it can cause pseudorubella, fever, pityriasis rosea, convulsions, and chronic fatigue syndrome.
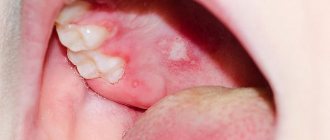
Herpes virus HHV-8 (HHV-8, Kaposi's sarcoma)
Clinical manifestations are observed only with immunosuppression. They are expressed in the appearance of vascular seals and form on the skin and in the mouth. These are multiple hemorrhagic neoplasms of a malignant nature. Over time, the virus can cause complications in the lungs, gall bladder, bile ducts and other internal organs.
Treatment
Since the herpes virus is integrated into the genome of a human cell, it cannot be removed. Therapeutic measures are aimed at diseases caused by herpes viruses. For treatment, etiotropic drugs and means to relieve symptoms are prescribed. The choice of medications, dosage and method of treatment are chosen individually, based on the patient’s condition, the presence of complications and concomitant diseases.
Herpes viruses are activated against a background of suppressed immunity; most patients are HIV-infected. In this case, treatment is indicated in immunologically favorable conditions, in which case the risk of complications is lower and the chances of cure are higher. However, patients with a poor prognosis also undergo individual, not always systematic, treatment.
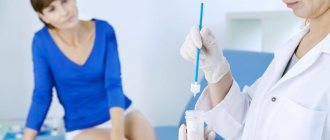
Diagnostics
Diagnosis of infections caused by herpes viruses is based on laboratory tests. They use polymerase chain reaction methods performed in real time.
PCR is the most accurate way to detect all types of herpes. It is also one of the fastest in production and allows identifying the pathogen at an early stage of the disease. To obtain the result, a small amount of the desired material is sufficient.
A special feature of the PRC method is the copying of a DNA region specific to the pathogen. It is reproduced a number of times that enlarges the sample to a size acceptable for diagnosis.
Any biological material is suitable as a sample:
- deoxygenated blood;
- seminal fluid;
- vaginal discharge;
- saliva;
- cerebrospinal fluid;
- biopsy and others.
Other methods for diagnosing herpes include molecular biological studies of skin biopsies for the diagnosis of Kaposi's Sarcoma. Hair follicles are examined to confirm or rule out HHV-6. The digital droplet PCR method helps to identify chromosomally integrated and inherited forms of type 6 virus. For tissue-invasive CMV, preference is given to histological and immunohistochemical studies.
When is a test for herpes viruses prescribed?
- In the presence of symptoms indicating diseases caused by herpes viruses.
- To differentiate herpesvirus and other infections.
- When planning pregnancy and in the presence of pregnancy pathology.
- For a comprehensive examination of HIV patients and people with immunodeficiency.
- Before immunosuppressive therapy.
- To monitor the effectiveness of therapeutic measures.
It is necessary to undergo a herpes test for patients with hematological malignancies and lymphoproliferative diseases. A study before and after transplantation is indicated.
Complications of cytomegalovirus infection
In most cases, complications with CMV infection are specific:
- pleurisy and pneumonia;
- myocarditis;
- arthritis;
- sensory disturbance;
- encephalitis.
Nonspecific complications are also observed, which are caused by the addition of a secondary bacterial infection. We are talking about purulent complications. In this case, the body temperature rises to 40-41 degrees, and a corresponding clinical picture of damage to internal organs appears.
Cytomegalovirus can cause miscarriage, stillbirth, or death of an infant immediately after birth. Take care of your child's health. Make an appointment with a doctor to promptly identify the presence of the disease in the body and treat it.
Epidemiology[edit]
More than 95% of adults are infected and immune to HHV-7 [35], and more than three quarters of them were infected before the age of six years. [36] Primary HHV-7 infection in children usually occurs between 2 and 5 years of age, meaning that it occurs after primary HHV-6 infection. [37] A 2014 Washington University School of Medicine analysis of 102 healthy adults sampled in five major habitats found that HHV-7 was present in 98% of them, especially in the mouth. [38] A 2022 study on the human blood virome of 8,240 people aged 2 months to 102 years found that 20.37% were positive for HHV-7. [39]
Pathogenesis (what happens?) during Cytomegalovirus infection
The entry point for Cytomegalovirus is the mucous membrane, mainly of the upper respiratory tract and oropharynx. Infection can also occur through the genitourinary system, gastrointestinal tract and other organs.
There are no structural changes observed at the injection site. After the virus enters the body, it begins to attack mono- and lymphocytes, as well as the epithelium of the salivary glands, kidneys, lungs and other internal organs. In this case, cytomegaly is observed (an increase in the size of the affected cells by 3-4 times). Immature virions are formed in the cell nuclei. As a result, the cellular structures take on the appearance of an “owl’s eye.” The active course of the disease leads to the development of depression of most parts of the immune system, including the IFN-α protein.
In response to the penetration of CMV, a protective reaction develops - specific antibodies are formed, and delayed-type hypersensitivity is observed. This process is accompanied by the appearance of nodular formations of lymphocytic infiltrates. Cells that have been infected continue to function, secreting a special secret that masks the virus from the protective functions of the human body.