Thiogamma 600 tablets p/o 600 mg No. 10x3
Name
Thiogamma 600 tablets, coated, 600 mg, blister pack No. 10x3
Description
Capsule-shaped, film-coated tablets, light yellow in color, with possible white inclusions, scored on both sides.
Main active ingredient
Thioctic acid
Release form
Pills
Dosage
600mg
pharmachologic effect
Thioctic (alpha-lipoic) acid is an endogenous antioxidant that functions as a coenzyme in the oxidative decarboxylation of alpha-keto acids. In diabetes mellitus, as a result of hyperglycemia, the content of advanced glycation end products increases. This process contributes to a decrease in endoneural blood flow and the development of endoneurial hypoxia. At the same time, along with an increase in the production of free radicals, the content of antioxidants, in particular glutathione, decreases. In clinical studies in patients with diabetic polyneuropathy, the administration of thioctic acid led to a decrease in sensory disturbances accompanying diabetic polyneuropathy, such as dysesthesia, paresthesia, pain, and numbness.
Indications for use
Paresthesia in diabetic polyneuropathy.
Directions for use and doses
The basis of treatment for diabetic polyneuropathy is optimal treatment of diabetes mellitus. For diabetic polyneuropathy in adults, the daily dose is 1 tablet per day (600 mg of thioctic acid), taken as a single dose on an empty stomach approximately 30 minutes before meals. The tablets are taken without chewing, with a small amount of liquid. The score on both sides of the tablet is intended only to facilitate patient administration and not to divide into doses. For severe polyneuropathy, treatment can be started with intravenous administration of thioctic acid. Concomitant food intake may interfere with the absorption of thioctic acid, so patients with a long gastric emptying time should take the tablets half an hour before breakfast. Because diabetic neuropathy is a chronic disease, long-term treatment may be required.
Use during pregnancy and lactation
Due to the lack of sufficient clinical data, the use of the drug during pregnancy is not recommended. There is no information on the penetration of thioctic acid into breast milk. A decision must be made to either stop breastfeeding or discontinue therapy, taking into account the benefits of breastfeeding for the child and the benefits of therapy for the mother.
Precautionary measures
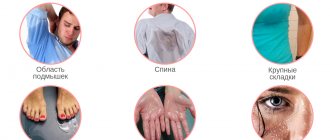
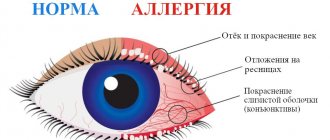
Regular alcohol consumption is an important risk factor for the onset and progression of polyneuropathy and may affect the results of treatment with thioctic acid. Patients taking Thiogamma® 600 should avoid drinking alcohol. In patients with diabetes mellitus, constant monitoring of blood glucose concentrations is necessary, especially at the initial stage of therapy. In some cases, it is necessary to reduce the dose of insulin or oral hypoglycemic drug to avoid the development of hypoglycemia. The medicine contains lactose; patients with hereditary galactose intolerance, lactase deficiency or glucose-galactose malabsorption should not take this medicine. Information for patients with diabetes: 1 tablet contains less than 0.0041 XE (bread units). Cases of autoimmune insulin syndrome (AIS) have been reported during treatment with thioctic (alpha-lipoic) acid. Patients with certain alleles, such as HLA-DRB1*04:06 and HLA-DRB1*04:03, are more susceptible to developing AIS when treated with thioctic acid. The HLA-DRB1*04:03 allele (predisposition to AIS, odds ratio: 1.6) is more common in Caucasians (more common in Southern Europe than in Northern Europe). The HLA-DRB1*04:06 allele (susceptibility to AIS, odds ratio: 56.6) is mainly found in individuals from Japan and Korea. Autoimmune insulin syndrome should be considered in the differential diagnosis of spontaneous hypoglycemia in patients receiving thioctic acid (see section "Side effects")
Interaction with other drugs
When Thiogamma® 600 is used simultaneously with cisplatin, the effect of the latter is reduced, because thioctic acid (alpha-lipoic acid) forms ionic complexes with metals. For these reasons, simultaneous use with iron and magnesium supplements, and with dairy products containing calcium is not recommended. When taking the daily dose 30 minutes before breakfast, iron and magnesium supplements can be taken at lunchtime or in the evening. When used simultaneously, thioctic acid enhances the effect of insulin and oral hypoglycemic agents. In some cases, it is necessary to reduce the dose of insulin or oral hypoglycemic drug to avoid the development of hypoglycemia. Alcohol (ethanol) reduces the therapeutic activity of thioctic acid. Patients taking the drug Thiogamma®600 should refrain from drinking alcohol, including during intervals free from taking the drug.
Contraindications
Hypersensitivity to thioctic acid or other components of the drug. Children and youth under 18 years of age (efficacy and safety of use have not been established).
Compound
1 film-coated tablet contains the active ingredient: thioctic acid - 600 mg; excipients: hypromellose, anhydrous colloidal silicon dioxide, microcrystalline cellulose, lactose monohydrate, carmellose sodium, talc, simethicone (consisting of dimethicone and colloidal silicon dioxide 94:6), magnesium stearate. Film shell composition: hypromellose, macrogol 6000, talc, sodium lauryl sulfate.
Overdose
Symptoms: nausea, vomiting, headache. In case of accidental or suicidal use of oral doses of 10 to 40 g of thioctic acid together with alcohol, severe intoxication may develop with a fatal outcome. The picture of a severe overdose is manifested by psychomotor agitation, impaired consciousness, generalized convulsions, and lactic acidosis. Hypoglycemia, shock, rhabdomyolysis, hemolysis, disseminated intravascular coagulation, bone marrow suppression, and multiple organ failure may result from this intoxication. Treatment is symptomatic. There is no specific antidote. Treatment of severe intoxication is carried out in the intensive care unit. Therapeutic measures If thioctic acid intoxication is strongly suspected (e.g., more than 10 tablets containing 600 mg thioctic acid in adults and more than 50 mg/kg in children), immediate hospitalization is necessary and measures taken in accordance with general methods of treating intoxications (e.g., induced vomiting, gastric lavage, taking activated charcoal, etc.). When treating attacks of generalized seizures, lactic acidosis and other life-threatening consequences of overdose, one should be guided by the principles of modern intensive care. The benefits of hemodialysis, hemoperfusion or hemofiltration methods for the forced elimination of thioctic acid have not yet been confirmed.
Side effect
When assessing side effects, the following gradation of the frequency of their occurrence is taken as a basis: - very often (> 1/10); - often (> 1/100 - 1/1000 - 1/10,000 -
Storage conditions
Store in a place protected from light at a temperature not exceeding 25°C. Keep out of the reach of children.
Buy Thiogamma 600 tablets p/o 600 mg No. 10x3 in the pharmacy
Price for Thiogamma 600 tablets p/o 600 mg No. 10x3
Instructions for use for Thiogamma 600 tablets p/o 600 mg No. 10x3
Thiogamma
The German drug thiogamma is a metabolic drug that controls the metabolism of lipids and carbohydrates and is used in the treatment of diabetic and alcoholic polyneuropathy (multiple lesions of areas of the peripheral nervous system). The pharmacologically active substance of the drug is thioctic acid. Diabetic polyneuropathy (polyneuropathy) is a typical complication of diabetes mellitus. It begins to manifest itself with numbness, burning and pain in the limbs at rest. Over time, this whole complex of unpleasant sensations gains intensity and begins to overwhelm the patient constantly. Polyneuropathy is characterized by movement disorders and reflex disorders. The first to become involved in the pathological process are thin sensory nerve fibers, the defeat of which entails a decrease or complete loss of temperature and pain sensitivity. Then comes the turn of thick nerve fibers, the “switching off” of which leads to disruption of proprioceptive (sensing the position of body parts relative to each other) and vibration sensitivity, inhibition of the spread of excitation. The “attack” of the disease on the motor nerves leads to atrophic changes in the small muscles of the foot and interosseous muscles, impaired muscle tone of the flexors and extensors of the fingers. The reason for such serious consequences of diabetes is prolonged and uncontrolled hyperglycemia. To prevent these complications, it is necessary to constantly maintain blood glucose levels within normal limits. But in addition to the pathogenetic therapy of diabetes, one cannot do without a symptomatic component, namely, the effect on damaged nerve fibers. In the treatment of diabetic polyneuropathy, the drug thiogamma based on thioctic acid, a coenzyme produced in the human body, has proven itself well. Thioctic acid provides transmembrane transport of glucose, participates in the process of formation of glucose from other compounds, thereby replenishing energy deficiency.
Regulates the metabolism of carbohydrates and lipids, stimulates cholesterol metabolism, improves liver function. It has a hepatoprotective effect and reduces the amount of “bad” cholesterol. Improves nervous trophism. Reduces the level of free radicals (antioxidant properties). A number of studies have demonstrated the hypoglycemic properties of thioctic acid. In type 2 diabetes mellitus, the amount of thioctic acid synthesized in the human body is significantly reduced. Its lack negatively affects energy metabolism. In such situations, it is almost impossible to do without replenishing the deficiency of this substance from external sources, which is the drug thiogamma. The results of recent clinical trials of this drug indicate normalization of glutathione concentrations in peripheral nerves, which has a beneficial effect on their conductivity. In addition, while taking the drug, the signs of oxidative stress that develops under the influence of free radicals and developed endoneural hypoxia are leveled. Thioctic acid is able to dissolve in both water and lipids, which allows it to be active in aqueous and fatty environments and is rightfully considered one of the most powerful antioxidants. This compound accumulates in large quantities in peripheral nervous tissue, normalizing the blood supply to nerve fibers and increasing the speed of excitation. Thiogamma is a well-studied drug with a favorable safety profile. Its initial daily dose should be 600 mg. The treatment tactics are as follows: first, thiogamma is administered intravenously for 2-4 weeks, then they switch to using the drug in tablet form at a dose of 600 mg once a day. For preventive purposes, thiogamma is prescribed in tablet form to take 600 mg of the drug once a day for a long time.
In recent years, the diagnosis and treatment of fatty liver disease not only remains relevant, but also becomes an important social problem. An unhealthy diet, addiction to fast food, hypercaloric foods, a sedentary lifestyle, as well as the habit of “eating” and chronic stress contribute to an increase in the incidence of metabolic syndrome (MS), which is based on insulin resistance (IR). In turn, non-alcoholic fatty liver disease (NAFLD) is the hepatic component of MS and is diagnosed if fatty inclusions are detected in the liver tissue in the absence of a history of long-term alcohol consumption in toxic doses.
The prevalence of NAFLD in the general population has not been studied, but it has been found that the disease is most often diagnosed in middle and old age [1, 2]. Women aged 40 to 60 years with signs of MS are at maximum risk of developing NAFLD [3]. According to 2009 data, NAFLD in Russia was detected among 27% of 30 thousand outpatients examined, with 16.8% diagnosed with non-alcoholic steatohepatitis and 2.9% with liver cirrhosis [4].
Risk factors for the development of NAFLD are obesity, type 2 diabetes mellitus, fasting, parenteral nutrition, the presence of ileocecal anastomosis, and bacterial overgrowth in the intestine, which, along with endotoxemia, plays an important role in the development of IR. The pathological reactions are based on the activation of Kupffer cells under the influence of a large number of bacterial antigens and toxins entering the liver with the blood flow through the portal vein. Activated cells synthesize pro-inflammatory cytokines, which in turn stimulate inflammation and collagen formation. In addition, pro-inflammatory cytokines (namely tumor necrosis factor α - TNF-α) activate phosphorylation of type 1 insulin receptors, which leads to a decrease in their sensitivity.
A change in the number and impaired sensitivity of insulin receptors lead to the development of a number of pathological systemic reactions. Thus, changes in the activity of enzymes of intracellular glucose metabolism (glycogen synthetase, pyruvate dehydrogenase) contribute to impaired carbohydrate metabolism in the form of changes in carbohydrate tolerance and/or the development of non-insulin-dependent diabetes mellitus. Impaired lipid metabolism manifests itself in the activation of the synthesis of free fatty acids (FFA) and triglycerides (TG), increased synthesis of apo-B-lipoproteins, changes in the synthesis or secretion of very low-density lipoproteins (VLDL), and a decrease in the rate of β-oxidation of FFAs [5].
An increase in the supply of FFAs to the liver due to their high content in food, release during lipolysis of adipose tissue, as well as the conversion of excess carbohydrates entering the liver into fatty acids is accompanied by the accumulation of fat in hepatocytes and stellate cells. Small droplets of fat in hepatocytes begin to be detected by light microscopy if its amount increases to 2–3%. This pathological condition is regarded as fatty infiltration (steatosis) of the liver. The main components of hepatocellular lipids are represented by TG, the substrates for the synthesis of which are fatty acids and glycerophosphate. The production of TG in the hepatocyte is directly dependent on the content of fatty acids, acetyl coenzyme A and glucose. Transport of TG from the cell occurs as part of VLDL. Conjugation of TG with apoproteins occurs on the surface membranes of the endoplasmic reticulum with the participation of a number of enzymes. As metabolic disorders progress, the synthesis and secretion of VLDL decreases, which leads to impaired TG excretion from liver cells. Fat vacuoles push back and compress the organelles of hepatocytes, which leads to damage, stretching and rupture of membranes. As fat accumulates, the liver cell becomes more vulnerable and sensitive to toxic influences [6–8].
The formation of the next stage of NAFLD – steatohepatitis – is characterized by inflammatory-necrotic changes in the liver. Most authors recognize the cause of inflammation as oxidative stress and increased lipid peroxidation (LPO) due to the induction of cytochrome P450 2E1. Reactive oxygen species in the form of free radicals lead to damage to hepatocytes: swelling of mitochondria and fragility of lysosomes, disruption of the integrity of cell membranes and the functioning of membrane-dependent transport systems of the hepatocyte. It is at this stage of disease development that hyperproduction of cytokines occurs, as well as secretion of hormone-like substances – adipokines – by adipocytes. The accumulation of toxic intermediate products against the background of severe hormonal disorders stimulates collagen formation and the development of fibrosis.
The clinical picture of NAFLD is poor and nonspecific. The disease can be asymptomatic for a long time and pathological signs from the liver are often discovered by chance - during examination for another pathology (diabetes mellitus, arterial hypertension, endocrine disorders). There may be no complaints from most patients. However, some patients experience manifestations of asthenic (increased fatigue, weakness) and dyspeptic (flatulence, nausea, stool disorders, heaviness in the right hypochondrium) syndromes. During physical examination, some patients have palpable enlarged liver to varying degrees. An echographic examination reveals fatty infiltration of the liver, the semiotics of which include a compact arrangement of echo signals, increased echogenicity of the liver tissue (“white liver”), depletion of the vascular pattern, and distal attenuation of the echo signal.
The biochemical picture of NAFLD is characterized by a moderately pronounced cytolytic syndrome - an increase in the activity of alanine and aspartic transaminases in a fairly wide range; in some patients there is an increase in the activity of markers of cholestasis - alkaline phosphatase and gammaglutamyl transpeptidase, usually not exceeding 2-3 norms. The level of bilirubin in patients with non-alcoholic steatohepatitis is usually within normal limits, the protein-synthetic function of the liver is preserved, the level of albumin and prothrombin decreases only with the development of cirrhosis and liver failure.
A simple and reliable method for assessing the presence and severity of IR is to determine HOMA-IR (HOmeostasis Model Assessment) using the formula: HOMA-IR = I0 x G0/22.5, where I0 is the fasting insulin level (μIU/ml), G0 is the glucose level on an empty stomach, (mmol/l). The presence of IR is indicated by a HOMA-IR value ≥ 2.7.
Due to the non-specificity of the clinical and biochemical picture, the diagnosis of NAFLD should be verified by the data of a morphological study of hepatobiopsy.
The following abnormalities are detected in the liver tissue of patients:
• fatty liver of varying degrees of severity (large-droplet, small-droplet, mixed);
• inflammatory infiltration (centrilobular, less often portal and periportal) with neutrophils, lymphocytes, histiocytes;
• fibrosis of varying severity (perihepatocellular, perisinusoidal and perivenular).
NAFLD is benign, however, according to dynamic morphological studies of liver tissue, approximately half of the patients experience increased fibrosis over time. According to the literature, the formation of liver cirrhosis occurs in 7–16% of adult patients.
Treatment of patients with NAFLD that developed as part of MS begins with the correction of its main manifestations. The primary measures should be aimed at reducing body weight: changing lifestyle, reducing caloric intake, increasing physical activity. It is important to remember that too rapid loss of body weight naturally leads to an increase in inflammatory activity and the progression of fibrosis. At the same time, a decrease in body weight by 11–20 kg/year (7–10% of the initial weight) has a positive effect on the severity of steatosis, inflammation and the degree of liver fibrosis. A safe level of weight loss (500–1500 g per week) is achieved with a daily calorie intake of 25 kcal/kg and vigorous exercise and/or use of the intestinal lipase inhibitor orlistat. Against the background of weight loss and normalization of biochemical parameters, a significant decrease in steatosis, inflammation and fibrosis of the liver is observed.
Taking into account the dominant role of oxidative stress in the development and progression of NAFLD, the use of drugs with antioxidant activity is recommended to correct metabolic disorders. Thiogamma (Wörwag Pharma GmbH and Co. KG, Germany) is considered such a drug, the active ingredient of which is the meglumine salt of thioctic acid. In the body, thioctic (α-lipoic) acid is synthesized during the oxidative decarboxylation of α-keto acids and is an endogenous antioxidant. By the nature of its biochemical effect, thioctic acid is close to B vitamins, participates in the regulation of lipid and carbohydrate metabolism, and helps normalize cholesterol metabolism. By participating in the oxidation of fatty acids in mitochondria, thioctic acid reduces the content of substrates for TG synthesis and prevents the development of fatty liver [9–11].
The most widely known mechanism of action of thioctic acid is antioxidant activity, which consists in the deactivation of free radicals and normalization of the function of the glutathione system [12, 13].
The next important proven mechanism of action of thioctic acid is hypolipidemic, which consists in reducing cholesterol synthesis and releasing fatty acids from adipose tissue.
The hypoglycemic effect of thioctic acid consists of stimulating the activity of glucose transporters and the process of intracellular glucose transport itself, as well as inhibiting gluconeogenesis and ketogenesis. Thioctic acid regulates metabolism, reducing the concentration of glucose in the blood, thereby helping to overcome IR. This property of thioctic acid is difficult to overestimate for patients with MS, in the pathogenesis of which impaired glucose utilization plays an important role.
Studies have also shown the anti-inflammatory effect of thioctic acid, which manifests itself due to the suppression of the activity of inflammatory cytokines (interleukin-1, TNF-α), which play a major role in the development of IR and the progression of inflammatory changes in liver tissue. Recommendations for the use of thioctic acid are based on the results of numerous foreign studies (ALADIN, ORPIL, NATHAN, DECAN, SYDNEY) [10, 11]. A meta-analysis of the results of these studies, which included 1258 patients, showed that intravenous administration of thioctic acid at a dose of 600 mg for three weeks significantly reduced neurological symptoms [10, 14]. In these studies, a particularly significant effect was obtained when thioctic acid was used by patients with non-insulin-dependent diabetes mellitus against the background of MS. This is the most important rationale for the use of Thiogamma in NAFLD, which develops as a result of disorders of carbohydrate, fat and protein metabolism in MS [15, 16].
Thus, based on the above-described properties of thioctic acid, it is obvious that the drug Thiogamma has the following pathogenetically substantiated actions that make it possible to correct the main metabolic disorders that develop in MS and NAFLD [9, 17]:
• insulin-like effect on transmembrane glucose transport [11], increases the rate of glucose transport into the cell, promoting the accumulation of glycogen in the cell;
• has a positive effect on cholesterol metabolism;
• has a beneficial effect on reparative processes (including in hepatocytes) and improves liver function [11];
• reduces inflammation, reducing the severity of lipid peroxidation, binding free radicals and free tissue iron;
• prevents the development of fatty liver infiltration;
• increases the energy supply of the cell and helps prevent the development of ketoacidosis;
• helps prevent and relieve rheological disorders and vascular disorders due to suppression of nitric oxide synthesis by hepatocytes.
The daily dose of the drug is 600 mg in one oral dose or in one infusion (slow administration of the drug at a rate of no more than 50 mg per minute). The decision on the duration of treatment is made by the attending physician, depending on the clinical and biochemical activity of the disease. However, taking into account the need to correct profound metabolic disorders, it is recommended that Thiogamma therapy be carried out for at least 2–4 months with possible repeated courses (as indicated). The drug has a good tolerability and safety profile; allergic reactions such as urticaria are rare. No teratogenic or carcinogenic effects of thioctic acid have been identified. A number of works by domestic and foreign authors indicate the effectiveness of Thiogamma in the complex treatment of NAFLD [12, 13].
Thus, the numerous systemic effects of thioctic acid on carbohydrate, fat and protein metabolism make the use of Thiogamma pathogenetically justified for fatty liver disease associated with IR and type 2 diabetes mellitus.