Article for the “Bio/Mol/Text” competition: Acne, or in common parlance pimples, is estimated to affect 70% of people and almost 85% of teenagers. Acne is a complex, multifactorial disease, and we became interested in the role of microorganisms that live on the skin. It is known that the skin of an unborn child is sterile, but soon after birth it is colonized by a variety of environmental microorganisms. They form the microbiome of our external integuments. We decided to take a closer look at whether bacteria and skin get along, what relationships are formed between bacteria - and how genetics and environment influence this.
Competition “Bio/Mol/Text”-2021/2022
This work was published in the “School” category of the “Bio/Mol/Text” competition - 2021/2022.
The nomination partner is the non-profit boarding school “Letovo”.
The general partner of the competition is the international innovative biotechnology company BIOCAD.
The general partner of the competition is: the largest supplier of equipment, reagents and consumables for biological research and production.
"Book" sponsor of the competition - "Alpina Non-Fiction"
Skin structure
Human skin consists of three layers (Fig. 1).
The hypodermis, or subcutaneous fat, is the deepest layer. This is the main store of fat in the body.
The dermis (or skin itself) is located between the epidermis and hypodermis. It is a connective tissue with numerous collagen and elastin fibers. It largely determines the condition of the skin - hydration and elasticity.
The dermis is in contact with the basal layer, which gives rise to the upper layer, the epidermis. The main cells of the epidermis are keratinocytes. As they grow, they move upward from the basement membrane, releasing lipids. There are several layers of keratinocytes, depending on the age and condition of the cells, which are replaced in about a month [1].
We have not yet mentioned epidermal derivatives. Sebaceous, sweat, sulfur and mammary glands, hair have the same origin. The sebaceous glands secrete sebum (sebum), which microorganisms happily feed on.
The skin is a separate ecological niche in which complex microbial communities are formed. The set of microorganisms inhabiting the skin, or microbiome, is a dynamic system with its own patterns. Here, as in any ecosystem, there are different connections between organisms. Microorganisms also interact with the environment, which largely dictates the conditions of their existence [2].
Figure 1A. Skin structure.
Figure 1B. The structure of the epidermis.
author's drawing
Figure 1B. The structure of the sebaceous gland.
Now let's talk about the skin barrier. This is a system of various lipids and keratinocytes that protects the skin from dehydration and mechanical damage. But within the framework of the article, we are interested in the fact that the skin barrier is needed to prevent the effects of bacteria and pathogens on the vital activity of skin cells [3].
The skin barrier is usually divided into two components - the hydrolipid mantle and the lipid-epidermal barrier (Fig. 2). The main functional feature of the hydrolipid mantle, consisting mainly of secretions of the sebaceous and sweat glands, is an acidic environment, pH approximately from 4.7 to 5.7. Thanks to it, harmful bacteria stop multiplying and die. The lipid-epidermal barrier consists of many dead cells held together by lipids, which they gradually released into the external environment as they grew older. It is this that protects the skin from dehydration, reducing transepidermal water loss (TEWL), and is also the second link of protection after the hydrolipid mantle from the penetration of bacteria and allergens into the skin.
However, we should not assume that the skin barrier completely protects us from pathogens. Research still revealed the presence of bacteria in the dermis and hypodermis [4].
Figure 2. The skin barrier does not completely protect the skin from bacterial penetration. As keratinocytes grow, they secrete numerous sheets containing lipids (keratinosomes). Thanks to intercellular ligaments - corneodesmosomes - and lipids, corneocytes, that is, dead cells of the stratum corneum of the epidermis, are attached to each other, forming an integral structure, and are moisturized by NMF - a natural moisturizing factor. These are molecules that help retain more water in the epidermis: free amino acids, urea, lactates, etc.
website
Dermis
The dermis is a complex, loose connective tissue consisting of individual fibers, cells, a network of blood vessels and nerve endings, as well as epidermal outgrowths surrounding the hair follicles and sebaceous glands. The cellular elements of the dermis are represented by fibroblasts, macrophages and mast cells. Lymphocytes, leukocytes, and other cells are capable of migrating into the dermis in response to various stimuli.
The dermis, making up the main volume of the skin, performs primarily trophic and supporting functions, providing the skin with such mechanical properties as plasticity, elasticity and strength, which it needs to protect the internal organs of the body from mechanical damage. The dermis also retains water, participates in thermoregulation and contains mechanoreceptors. Finally, its interaction with the epidermis supports the normal functioning of these layers of skin.
In the dermis there is no such directed and structured process of cell differentiation as in the epidermis, however, it also exhibits a clear structural organization of elements depending on the depth of their occurrence. Both the cells and the extracellular matrix of the dermis also undergo constant renewal and remodeling.
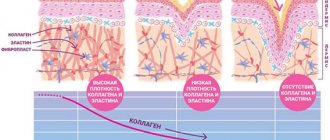
The extracellular matrix (ECM) of the dermis, or intercellular substance, which consists of various proteins (mainly collagen, elastin), glycosaminoglycans, the most famous of which is hyaluronic acid, and proteoglycans (fibronectin, laminin, decorin, versican, fibrillin). All these substances are secreted by dermal fibroblasts. The ECM is not a random accumulation of all components, but a complexly organized network, the composition and architectonics of which determine such biomechanical properties of the skin as rigidity, extensibility and elasticity. Epidermal keratinocytes, which are closely connected to each other, are attached to the ECM proteins. They form a dense protective layer of the skin. The structure of the ECM is also capable of exerting a regulatory effect on the cells immersed in it. Regulation can be either direct or indirect. In the first case, ECM proteins and glycosaminoglycans directly interact with cell receptors and initiate specific signal transduction pathways in them. Indirect regulation is carried out through the action of cytokines and growth factors retained in the cells of the ECM network and released at a certain moment to interact with cell receptors. The ECM structural network is subject to remodeling by enzymes from the matrix metalloproteinase (MMP) family. In particular, MMP-1 and MMP-13 initiate the degradation of collagen types I and III. The density of the ECM network of the dermis is uneven - in the papillary layer it is looser, in the reticular layer it is much denser, both due to the closer arrangement of the fibers of structural proteins and due to the increase in the diameter of these fibers.
Collagen is one of the main components of the ECM of the dermis. Synthesized by fibroblasts. The process of its biosynthesis is complex and multi-stage, as a result of which the fibroblast secretes procollagen into the extracellular space, consisting of three polypeptide α-chains folded into one triple helix. Procollagen monomers are then enzymatically assembled into extended fibrillar structures of various types. In total, there are at least 15 types of collagen in the skin; in the dermis, types I, III and V of this protein are most abundant: 88, 10 and 2%, respectively. Type IV collagen is localized in the basement membrane zone, and type VII collagen, secreted by keratinocytes, plays the role of an adapter protein for attaching ECM fibrils to the basement membrane (Fig. 4). Fibers of structural collagens of types I, III and V serve as a framework to which other ECM proteins are attached, in particular collagens of types XII and XIV. It is believed that these minor collagens, as well as small proteoglycans (decorin, fibromodulin and lumican), regulate the formation of structural collagen fibers, their diameter and the density of the formed network. The interaction of oligomeric and polymeric collagen complexes with other proteins, ECM polysaccharides, various growth factors and cytokines leads to the formation of a special network with certain biological activity, stability and biophysical characteristics important for the normal functioning of the skin. In the papillary layer of the dermis, collagen fibers are located loosely and more freely, while its reticular layer contains larger strands of collagen fibers.
Rice. 4. Schematic representation of skin layers and distribution of different types of collagens.
Collagen is constantly renewed, degrading under the action of proteolytic enzymes collagenases and being replaced by newly synthesized fibers. This protein makes up 70% of the skin's dry weight. It is the collagen fibers that “withstand the blow” under mechanical influence on it.
Elastin forms another network of fibers in the dermis, giving the skin such qualities as firmness and elasticity. Compared to collagen, elastin fibers are less rigid and curl around collagen fibers. It is with elastin fibers that proteins such as fibulins and fibrillins bind, to which, in turn, latent TGF-β-binding protein (LTBP) binds. Dissociation of this complex results in the release and activation of TGF-β, the most potent of all growth factors. It controls the expression, deposition and distribution of collagens and other matrix proteins in the skin. Thus, the intact network of elastin fibers serves as a depot for TGF-β.
Hyaluronic acid (HA) is a linear polysaccharide consisting of repeating dimers of D-glucuronic acid and N-acetylglucosamine. The number of dimers in the polymer varies, which leads to the formation of HA molecules of different molecular weights and lengths - 1x105-107 Da (2-25 μm), which, accordingly, have different biological effects.
HA is a highly hydrophilic substance that affects the movement and distribution of water in the dermal matrix. Thanks to this property, our skin, normally and in youth, has high turgor and resistance to mechanical pressure.
HA easily forms secondary hydrogen bonds both within one molecule and between neighboring molecules. In the first case, they ensure the formation of relatively rigid spiral structures. In the second, association with other HA molecules and nonspecific interaction with cell membranes occurs, which leads to the formation of a network of polysaccharide polymers with fibroblasts included in it. Shorter molecules of proteoglycans (versican, lumican, decorin, etc.) “sit” on a long HA molecule, like on a thread, forming aggregates of enormous sizes. Extended in all directions, they create a scaffold, contributing to the stabilization of the ECM protein network and fixing fibroblasts in a specific matrix environment. Taken together, all these properties of HA endow the matrix with certain chemical characteristics - viscosity, density of “cells” and stability. However, the ECM network is a dynamic structure that depends on the state of the organism. For example, under conditions of inflammation, HA aggregates with proteoglycans dissociate, and the formation of new aggregates between newly synthesized HA molecules (renewed every 3 days) and proteoglycans is blocked. This leads to a change in the spatial structure of the matrix: the size of its cells increases, the distribution of all fibers changes, the structure becomes looser, the cells change their shape and functional activity. All this affects the condition of the skin, leading to a decrease in its tone.
In addition to regulating water balance and stabilizing the ECM, HA plays an important regulatory role in maintaining epidermal and dermal homeostasis. HA actively regulates dynamic processes in the epidermis, including keratinocyte proliferation and differentiation, oxidative stress and inflammatory response, maintenance of the epidermal barrier and wound healing. In the dermis, HA also regulates fibroblast activity and collagen synthesis. By remodeling the matrix, HA controls the functioning of cells in the matrix, influencing their availability to various growth factors and changing their functional activity. Cell migration and the immune response in tissue depend on the action of GC. Thus, changes in the distribution, organization, molecular weight and metabolism of GCs have significant physiological consequences.
Fibroblasts are the main type of cellular elements of the dermis. It is these cells that are responsible for the production of HA, collagen, elastin, fibronectin and many other intercellular matrix proteins necessary for the formation of connective tissue. Fibroblasts in different layers of the dermis differ both morphologically and functionally. Not only the amount of collagen they synthesize depends on the depth of their location in the dermis, but also the ratio of the types of this collagen, for example types I and III, as well as the synthesis of collagenase: fibroblasts in the deeper layers of the dermis produce less of it. In general, fibroblasts are very plastic cells, capable of changing their functions and physiological response and even differentiating into another cell type depending on the stimulus received. The role of the latter can be played by signaling molecules synthesized by neighboring cells and the restructuring of the surrounding ECM.
Pathogenesis of acne
The main mechanism of acne is an increase in sebum production and the number of differentiating keratinocytes under the influence of androgens. As a result, the pores become clogged, an anaerobic environment occurs, and aggressive strains of P. acnes begin to actively multiply. Sooner or later our body notices this, complex inflammatory mechanisms are launched, and acne occurs [5].
But not everything is so simple: acne, as already mentioned, is a multifactorial disease, and there are quite a few factors that can trigger it:
- Genetic features. There are several hereditary forms of acne. For example, those associated with the AR gene (androgen receptor). Sometimes acne is not due to excess androgens, but to hypersensitivity of the androgen receptors.
- Neuroendocrine disorders.
- Nutrition.
- Excess iodine and bromine, vitamin B12[5].
- Some drugs and chemicals (about 210 known).
- Use of broad-spectrum antibiotics. (Previously, acne could be treated this way). They acted against bacteria, but not against fungi of the genus Malassezia, the secretions of which also irritate the sebaceous glands [5].
- Smoking.
- Stress [5]. Although often the very fact of having acne and the inability to get rid of it is stressful.
- Photodamage.
- Incorrectly selected cosmetics.
How can care affect skin condition?
The surface of healthy skin has a slightly acidic environment. Under the influence of cosmetics, the pH changes, which changes the living conditions of the microflora, and can also disrupt the epidermal barrier.
A significant part of cleansers alkalize the surface of the skin. The lipids in the skin barrier are simply washed away. You can use an acidic tonic to minimize changes. But not everyone knows about this, and not all tonics have the appropriate pH. With an intact epidermal barrier, the skin can restore balance in a couple of hours, and a single exposure does not cause much harm.
There are also means that are harsher in relation to the microbiome. For example, chemical peels are a popular cosmetic procedure. Its essence comes down to the fact that the top layer of epidermal cells is removed, and the skin becomes smoother. However, along with these cells, many bacteria also leave, which reduces their species diversity. And diversity is often a sign of a good microbiome. Peeling also excessively lowers the pH of the skin.
Why is this important to know?
Many mass-produced cosmetics aimed at treating acne can, on the contrary, harm your skin. Therefore, it is important to carefully select cosmetics for home use [6]. Indeed, with systematic violation of the integrity of the epidermal barrier, the skin becomes more vulnerable to various external influences, and transepidermal moisture loss increases. This has a bad effect on both appearance and health.
Figure 3. Distribution of bacterial families on human skin. In the table below, the names of bacterial families are indicated under different colors; they correspond to the colors in the diagrams on the main diagram. Next to the diagrams are multi-colored dots: blue are areas with increased sebum production, green are wet, red are dry.
[7]
Risk factors
The etiology of pyoderma is not limited to the mechanisms of formation of the purulent-inflammatory process. Dermatologists know the forms of predisposition to this disease associated with lifestyle, heredity and the patient’s individual history.
Known risk factors:
- Failure to comply with personal hygiene rules. Constant skin contamination increases the risk of infection.
- Overheating or hypothermia of integumentary tissues. Temperature changes disrupt the functions of local immunity. Often, after hypothermia, the skin becomes a gateway to infection.
- Age. In newborns and the elderly, pyoderma most often occurs due to insufficient effectiveness of local immunity.
- The presence of a large number of allergic reactions and autoimmune diseases (especially in children.)
- Pathologies of the endocrine system. The condition of the skin largely depends on hormonal influences.
- Metabolic diseases: diabetes and obesity.
- Congenital and acquired immunodeficiency conditions.
- Poor nutrition leading to a deficiency of certain vitamins and microelements in the body.
- Unfavorable family heredity. Skin diseases may be associated with the transmission of genetic mutations.
The causative agents of pyoderma dermatitis are found in the environment and on the surface of the integumentary tissue. These are staphylococci, streptococci and some pathogenic fungi. People often become infected with pyogenic bacteria in a hospital setting.
Skin microbiome
Map. Who lives where
Let's look at the most common microorganisms and their habitats on the skin. Those of them who live in the same place on the body have the greatest influence on each other.
P. acnes and P. granulosum are found in areas rich in sebaceous glands. P. acnes predominates on the scalp, forehead, ears, back and sides of the nose. It is the main colonizer of the sebaceous glands.
Moist areas such as the navel or armpit mainly contain Staphylococcus and Corynebacteria species. The axillary region is populated by gram-positive bacteria of the genera Staphylococcus, Micrococcus, Corynebacterium, as well as some Propionibacterium (Fig. 3) [7].
Drier areas are predominantly inhabited by Staphylococcus, Micrococcus, Corynebacterium, Enhydrobacter and Streptococcus
Perhaps the name P. acnes we mentioned earlier made you think that it is these bacteria that cause acne.
First of all, it should be remembered that acne is a complex and multifactorial disease that cannot depend on one bacterium. Some studies even show that healthy skin follicles provide a habitat that allows only P. acnes to colonize, while Staphylococcus epidermidis and other species have been found in acne patients. Therefore, the role of P. acnes is quite ambiguous, and there is even a possibility that P. acnes does not cause acne, but, on the contrary, protects against it [8].
The opinion that the skin is a habitat only for bacteria is wrong. In fact, the microbiome of our integuments is much more diverse and includes various types of protists, fungi, and sometimes even animals like Arthropods! Below you can see the demodex skin mite, a typical inhabitant of human skin that feeds mainly on sebum. This comrade itself is harmless, but its excessive reproduction in some people can cause an allergic reaction, manifested in the form of an acne-like condition (small pimples).
Video 1. This is a skin mite. Every person has at least one species.
What are the types of skin diseases in humans?
Skin diseases can have different origins. They all differ in their appearance, symptoms and cause of formation.
The most common of them:
- Fungi. Fungal diseases of the skin in humans are usually caused by parasitic fungi, whose origin is plant-based. Such diseases usually affect: the nail plate, hair, and skin. Fungal diseases are contagious, which means they can easily be transmitted from one organism to another.
- Ulcers. The causative agents of pustular skin diseases are staphylococci and streptococci. Also, the cause of the formation of ulcers can be infections as a consequence of cooling and mental trauma. Pustular skin diseases are divided into two main types: superficial pyoderma and deep pyoderma.
- Skin diseases that are caused by animal parasites. Such diseases include: lice and scabies. The first one is quite easy to cure. The causative agent of scabies is usually a scabies itch or mite. At the first symptoms of scabies, it is necessary to begin treatment, as otherwise it can lead to the formation of eczema.
- Ringworm. There are several types of lichens. The most common of them are: lichen rosea, lichen planus, herpes zoster, and lichen cauliflower. Each species has its own characteristics and reasons for formation.
- Diseases of the skin glands. The most common types of these diseases are: seborrhea and acne. If seborrhea affects the head, then hair loss begins. Acne most often appears at a young age, most often based on seborrhea.
How bacteria adapted to live on the skin
The skin microbiome is formed under the influence of a wide variety of variables: age, skin pigmentation, environmental factors (sun rays, cosmetics that we constantly use) [7], [9], [10].
We have already looked at the distribution of species on the surface, let’s dig deeper. It was revealed that the lower part of the stratum lucidum of the epidermis (Fig. 1) has an alkaline reaction, the middle part has a neutral reaction, and already dead cells have an acidic reaction [1]. Thus, environmental conditions depend not only on the location on the body, but also on the depth at which bacteria colonize the skin.
All organisms strive to increase their numbers, and bacteria are no exception. Here are some of the successful strategies they developed:
- Variable proteins. Bacteria of the same species are different from each other, and the immune system is less able to recognize and deal with them.
- Proteins that provide attachment to the epithelium. The better the bacteria attach to the epidermis, the more this species will be present [11].
- Substances that prevent competitors from living: antibiotics, SCFAs (short-chain fatty acids), and so on [12].
- Substances that act against other strains of the same species [13].
A number of SCFAs are naturally produced by skin cells, as well as by beneficial bacteria, which have also learned to break down fatty acids into shorter fragments. Such bacteria help the skin perform its protective function, since SCFAs slow down the development of random microorganisms (beneficial bacteria mostly need this ability in order to take root on the skin). In our case, P. acnes ferment glycerol to various SCFAs, including propionic acid [12].
Symptoms
The external manifestations of pyoderma depend on the depth of damage to the skin, the causative agent of the infection and other factors. Many dermatitis manifests itself in several types of skin formations at once. Additional symptoms may be due to the underlying cause of the pathology and the effect of bacterial toxins on the body.
Symptoms of body intoxication:
- dizziness and weakness;
- nausea and vomiting;
- sleep disturbance;
- increased body temperature;
- decreased appetite;
- headache;
- chills.
If the symptoms listed above appear, you should immediately consult a doctor. The infection that causes pyoderma can spread through the blood and damage internal organs. Self-removal of skin lesions increases the risk of sepsis.
Do bacteria and our immunity get along?
Bacteria can activate various signaling pathways in human skin cells (Fig. 4).
Figure 4. The effect of microorganisms can be positive, negative or neutral. The relationship between bacteria and skin is exemplified by the skin's innate ability to detect microorganisms through Toll-like receptors (TLRs). Other types of interactions are shown in the figure: at the top - the influence of ordinary skin bacteria, at the bottom - pathogenic. On a colored background - descriptions of molecules. The scheme is quite conventional and there are much more real interactions. And they are more complicated.
author's drawing
Bacteria are able to influence the innate immune response of keratinocytes, making them more or less reactive [14].
Treatment
Typically, skin diseases are treated in the following ways:
- Following a diet and proper nutrition, taking the necessary vitamins.
- Treatment with medications to boost the immune system.
- Use of antibiotics if the skin disease has become severe.
- External treatment with ointments and creams.
Here you can read in detail how to treat foot fungus.
Are the microbiome and acne connected?
We have already understood that various bacteria live on the skin, which can be both beneficial and pathogenic. Now we should consider their connection with acne.
So, bacteria and fungi colonize the skin because it is a convenient habitat for them, since it has a suitable food source - for example, some kind of epidermal lipids. And here we come to an extremely interesting moment. This lipid is released to the surface from the cell under the control of genes. In conclusion: what is the true cause of acne - a pathogenic bacterium or genes? The influence of genes or environment (i.e., the established microbiome)?
Is R. acnes good or bad for health?
We previously mentioned P. acnes and promised to look into it in more detail. In this part of the article we will talk about beneficial and harmful traits, various interactions of this bacterium, as well as the possibility of determining it on yourself.
Useful features
P. acnes shares properties with dairy propionibacteria, which are considered harmless - and even beneficial [12]. Organisms like it have many positive properties: synthesis of vitamin B12, riboflavin, folic acid. P. acnes is also able to regulate the number of other bacteria by producing SCFAs (short chain fatty acids). Fermentation of glycerol by P. acnes and S. epidermidis can inhibit the growth of the most common skin pathogen, Staphylococcus aureus. This prevents the dangerous bacterium from colonizing skin wounds [15].
Opportunistic traits
The pathogenesis of acne is the mechanism of disease development in which P. acnes plays a significant role. It secretes enzymes that destroy human tissue components and can aggravate a number of other diseases [16], [17].
A clogged sebaceous gland creates an anaerobic environment. It is beneficial to P. acnes and promotes overgrowth. But during the development of acne, not only this bacterium was found there, but also other microorganisms, including S. epidermis. That is, more than just P. acnes is involved in the process.
The relationships between bacteria are ambiguous. S. epidermidis breaks down fatty acids into shorter SCFAs. It turns out that it can curb the excessive growth of P. acnes and indirectly improve the situation with acne [18]. Although not so long ago we talked about how S. epidermidis and P. acnes together “protect” us from Staphylococcus aureus (Staphylococcus aureus).
Interactions between P. acnes and other bacteria
P. acnes and related species can produce bacteriocins (substances that cause damage to bacterial cell structures). They usually act against closely related species, but at least one of them (propionicin) inhibits even some gram-negative bacteria and fungi. In addition, P. acnes is capable of producing 2 types of thiopeptide antibiotics (high sulfur content). They strongly inhibit protein synthesis in gram-positive bacteria. And some strains of P. acnes are able to suppress other strains of their own species! [13]
Bacteriocins and bacteriocin-like substances of P. acnes may be responsible for successful competition with other microorganisms, which explains its dominant presence in healthy follicles.
Interaction between P. acnes and skin cells
How else to explain the dominance of P. acnes? She has identified 4 proteins that can trigger the body's immune response. Some of them are variable (DsA1 and DsA2). It turns out that within the same species, bacteria are slightly different, as if they are camouflaged. This is how P. acnes evades immunity.
There are TLR9 receptors inside keratinocytes that are capable of sensing bacterial DNA. When P. acnes binds to them, a very interesting thing happens: the bacterium is, as it were, turned a blind eye and can thus exist inside the cell for several weeks. After which inflammation still starts [19].
What happens to healthy skin and skin with acne?
By looking at the proteins, you can quite accurately say which “development program” the cell followed. So, in the sebaceous glands of healthy skin there are many proteins that are involved in protection against cellular stress and reactive oxygen species.
However, the sebaceous glands of people with acne have discovered proteins that are involved in tissue repair. This indicates that something has gone wrong and the tissue has been damaged. The foreign proteins identified were exclusively from P. acnes. The most common are CAMP factors (about them in the next section), as well as proteins that ensure the attachment of bacteria to the epithelium.
It would seem that everything is quite clear: P. acnes in this case behaves like an aggressor. But it was not there! It turns out that these same P. acnes proteins are also found on healthy skin. This once again confirms the controversial role of P. acnes in the development of acne [11].
Diagnostics
To undergo the examination, you must make an appointment with a dermatologist. The doctor will ask the patient about complaints and study medical history to identify risk factors for the disease. The next stage of diagnosis is an initial examination of damaged skin. The dermatologist evaluates the shape and size of the ulcers, and also determines the depth of tissue damage. To clarify the cause of pyoderma and the severity of the patient’s condition, instrumental and laboratory examinations are prescribed.
Additional diagnostic methods:
- Dermatoscopy is a method of visual examination of areas of tissue damage. To determine the type of disease, the doctor uses an optical or digital device that allows you to repeatedly enlarge the image (photo). Dermatoscopy is used to search for specific signs of different types of pyoderma and differential diagnosis.
- Microbiological examination of the contents of blisters, boils and other pathological structures. The doctor carefully punctures the membrane of the bladder and collects the exudate in a sterile container. In the laboratory, specialists inoculate the material on various nutrient media to identify the causative agent of the disease. After specifying the type of causative agent of dermatitis, specialists conduct a test for the sensitivity of microorganisms to antibiotics. Bacterial culture is the most reliable way to diagnose the infectious form of the disease.
- Blood analysis. In the treatment room, a nurse collects venous blood and sends the material to the laboratory. First of all, specialists evaluate the ratio and quantity of blood cells. An immunological test can detect signs of an autoimmune disease. Also, if necessary, serological diagnostics are carried out: specialists look in the blood for specific antibodies produced by the body in response to infection. It is often necessary to exclude a sexually transmitted infection.
In case of nonspecific symptoms, the doctor must exclude the presence of other diseases with similar symptoms. For this purpose, differential diagnosis of toxicerma, epidermolysis bullosa, pemphigus vegetans and fungal skin lesions is carried out. The presence of HIV infection is excluded. If necessary, a consultation with an immunologist, allergist, rheumatologist and infectious disease specialist is scheduled.
Metabolic activity of P. acnes
Involvement in inflammation
A number of in vitro studies have shown that certain strains of P. acnes are able to trigger inflammation in skin cells through different mechanisms [19–22], while other strains are associated with healthy skin [23], [24]. Analysis of the P. acnes genome has revealed putative differences between them that may help explain its dual role [25].
It is clear that there are also proteins that are encoded in the genomes of all P. acnes, for example, CAMP (Christie-Atkins-Munch-Peterson) factors (5 in total). Inhibition of CAMP2 has been shown to attenuate inflammation in a mouse ear model. Their exact role in vivo is unclear, but they appear to be quite important since they are conserved in all P. acnes.
Prevention
Preventing infectious and inflammatory skin diseases on your own is not a difficult task.
Basic methods of prevention:
- hygienic skin care;
- timely treatment of cuts and burns;
- treatment of chronic infections;
- regular examinations by a dermatologist for chronic skin diseases;
- control blood sugar levels in diabetes.
Consultation with a dermatologist and immunologist will help the patient learn more about methods of prevention and treatment of pyoderma.
conclusions
The variety of microorganisms on our body is enormous. You can imagine the skin as a giant country whose population is constantly changing under the influence of the outside world, the immune system and interactions with neighboring countries. That is, with the microbiome of the surface of the eyes, intestines, and mucous membranes. The totality of genomes and metabolic pathways of some microbial symbionts can work for the benefit of our body [26], because some bacteria are capable of carrying out reactions beneficial to humans. Such representatives of the microbiome can be assessed as another human organ [27].
Changes within our body adjust the living conditions of bacteria. Thus, with increased secretion of sebum, changes in its qualitative composition occur.
The microbiome is a complex dynamic system. If we more fully characterize the relationships within it, it may provide additional insight into modern human evolution. Helps assess whether and how evolving technologies and lifestyle changes affect health and susceptibility to disease. In particular, to acne.
It is now known for sure that quantitative and qualitative changes in the microbiome complicate the course of acne. P. acnes secretions are thought to contain several proteins that may cause inflammation. However, since acne is a multifactorial disease, it is worth taking into account other causes, such as care, genetics, stress, nutrition, etc. In addition, P. acnes has a number of useful features: it “protects” us from S. aureus by acting as a role of a probiotic. So it is incorrect to blame her for everything.
Studies have begun to appear showing that the introduction of normal flora preparations improves skin condition (Aspergillus, Bifida Ferment Lysate, Lactobacillus/Rye Flour Ferment Filtrate). They show that one of the promising directions in the treatment of acne [28] (and not only) can be pro- and prebiotics, and drugs based on them.
Due to the complex systems of interaction between micro- and macroorganisms, the result is not always predictable. Studying the function of the microbiome in health and disease is an urgent task in medicine. And there are still many questions to be answered.
Thanks for reading the article! We will be happy to answer your questions in the comments.