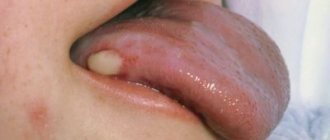
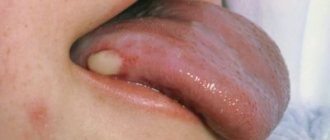
Pemphigus symptoms
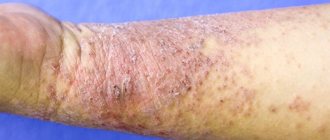
The characteristic symptoms of pemphigus are a rash of small blisters with serous contents. Their localization depends on what form of the disease develops:
- ordinary - rashes appear on the mucous membrane, accompanied by an unpleasant odor;
- leafy – blisters form on the skin, and crusts form at the same time;
- non-acantholytic - rashes are located on the mucous membrane and lips. This form of pathology is more often diagnosed in old age.
With pemphigus in children and adults, the following are also observed:
- severe weakness;
- increased drowsiness;
- deterioration or complete loss of appetite;
- losing weight even while eating high-calorie foods.
To detect pemphigus in newborns, older children and adults, the following examination methods are used:
- examination of the patient and assessment of rashes;
- Nikolsky test - with its help, differential diagnosis with other skin diseases is carried out;
- histological and cytological examination.
The symptoms of pemphigus (the second name of the disease) are often similar to the symptoms of other pathologies that will require completely different therapy. Therefore, before prescribing treatment, a thorough study of the symptoms that have arisen will be required.
1.General information
Acantholysis is one of the degenerative processes in the skin, manifested in the destruction of intercellular connections in the spinous layer of the epidermis, as a result of which the cells lose their cytoplasm and multiple vesicular formations appear on the skin. Further, acantholytic pemphigus is called “true” to emphasize its difference from many other dermatological diseases, which in medicine or in everyday speech are also called pemphigus.
True pemphigus, or pemphigus, belongs to the group of bullous dermatoses. To this day, much remains unclear in the etiology, pathogenesis and epidemiology of pemphigus, although the flow of research and publications on this topic does not dry out. In particular, it is still unknown why the disease is more common among representatives of Semitic and Mediterranean peoples, why females predominate among patients, why the highest incidence occurs in the age range of 40-60 years, etc.
A must read! Help with treatment and hospitalization!
Autoimmune pemphigus is an autoimmune bullous dermatosis that occurs due to the presence of autoantibodies (AAT) to antigens of the intercellular binding substance of stratified squamous epithelium, resulting in loss of connections between keratinocytes (acantholysis) with the subsequent formation of intraepidermal blisters [1, 2]. Pemphigus has all the signs characteristic of autoimmune diseases, which were formulated by immunologists back in the 40s of the 20th century [3]: the existence of specific AATs, the appearance of identical skin lesions in newborns from women suffering from pemphigus, combination with other autoimmune diseases and the existence of a special active genetic region [4].
AAT and target antigens in autoimmune pemphigus
AATs of autoimmune pemphigus have high tissue specificity and belong to IgG. They do not react with any of the antigens of tissues of other organs, except for the antigens of the intercellular binding substance (ICS) of the epithelium and Hassal bodies of the thymus of humans and animals [4-6]. The pathogenetic role of these AATs has been confirmed in animal experiments. Clinical signs of the disease were reproduced by intraperitoneal administration of sera containing IgG antibodies from patients with pemphigus [7]. In in vivo
with newborn BALB/c mice, it was demonstrated that with intraperitoneal administration of IgG antibodies from patients with an active form of the disease, clinical signs of the disease and AAT binding in the MCC of the mouse skin epithelium were observed in animals.
Administration of IgG from patients in long-term remission, from healthy relatives and controls did not cause clinical symptoms of the disease. This indicates that the final clinical manifestation of the disease is a consequence of the involvement of AAT [8, 9].
acantholysis was demonstrated in vitro by culturing human skin supplemented with IgG from the serum of patients with pemphigus vulgaris
Determination of IgG subclasses in pemphigus is an interesting and important point in the study of the disease. Many studies have shown that patients in the active stage of autoimmune pemphigus have high titers of IgG1 and IgG4, which indicates their pathogenetic role in this disease [10-12]. Other authors [10, 13] indicate that the humoral response in the active stage of pemphigus vulgaris is most often determined by the IgG4 subclass, and in the stage of remission - by IgG1.
In addition, an interesting fact was revealed: if IgG1 was detected only in the early stages of clinical manifestations of autoimmune pemphigus, then IgG4 was detected throughout the entire period of the disease [14]. These results suggest that the transition from the preclinical to the clinical stage of the disease, as well as from the stage of remission to the active process, is closely associated with the transition of subclasses from IgG1 to IgG4. In addition, the results show that IgG1 antibodies by themselves are not able to cause the disease. Thus, M. Lin et al. [12, 15] found that IgG1 antibodies from a healthy person who has only an IgG1 response are not capable of causing symptoms of the disease in an experimental model (mouse) when passively transmitted at a dose of 15 mg/kg, and IgG4 antibodies from patients with pemphigus are capable of triggering development of the disease when animals were given small doses of IgG4 antibodies (1.5 mg/kg), the concentration of which was 10 times less than when IgG1 antibodies were administered.
Some authors [16-19], when studying the pathogenesis of pemphigus, did not note a relationship between age, gender, severity or degree of disease activity and the type of IgG subclasses. Thus, when studying skin sections of patients in the active stage of pemphigus vulgaris by direct immunofluorescence, it was noted that IgG1 and IgG4 are the most common subclasses, but a pronounced immunohistochemical pattern was observed only with IgG4. Most patients in remission show deposition of IgG1 and IgG4 antibodies. It is extremely rare that both IgG1 and IgG4 are negative [18].
With different forms of pemphigus, the level and intensity of Ig fixation and deposition of immune complexes in the skin epithelium are different. The most intense fixation of antibodies in the epidermis is observed in pemphigus vulgaris, if the target antigen is desmoglein 3 [4, 20-22]. In pemphigus vulgaris and pemphigus vegetans, fixed Ig is localized in the intercellular spaces of predominantly the basal and spinous layers, in cases of pemphigus foliaceus and seborrheic pemphigus - in the intercellular substance of the spinous and predominantly granular layers of the epidermis, in paraneoplastic pemphigus - in the intercellular substance of all layers of the epidermis [4]. Fixed IgG can also be found in the cytoplasm of acantholytic cells and elements that make up the bottom or cover of the bladder. This phenomenon is caused by endocytosis of free immune complexes or impregnation of dead keratinocytes with them [5].
The localization of antigens to which antibodies are directed coincides with the localization of primary destructive changes in the epidermis. Tissue-bound Ig and immune complexes, determined by immunofluorescence, are detected both in areas of clinically affected skin and in areas without visible lesions, as well as during periods of exacerbation or remission of the disease in the absence of skin rashes, indicating a systematic tissue reaction [4] .
It has now been established that the targets for AAT, in addition to adhesion molecules and desmosomal glycoproteins, can be cadherins (desmogleins and desmocollins), plakoglobin, envoplakin and periplakin. In recent years, two new antigens (pemfaxine and α9-acetylcholine receptor) from the group of cholinergic receptors that are present on the surface of keratinocytes have been identified [4].
AAT or autospecific B cells themselves are not capable of causing the development of an autoimmune process. Regulatory disorders in the immune system play an important role in the pathogenesis of autoimmune diseases, including autoimmune pemphigus. However, even in the presence of such regulatory disorders, autoreactive cells can be in a state of immunological tolerance, and the onset of the autoimmune process is initiated by pathogenic, exogenous and/or endogenous factors [23].
Genetic predisposition and trigger factors for autoimmune pemphigus
In the pathogenesis of autoimmune pemphigus, genetic predisposition plays a major role. Many studies have found pemphigus AAT in first-degree relatives of patients. Thus, a number of authors [24–26], using immunofluorescence and immunoblotting methods, in 50% of cases noted a low level of AAT of pemphigus vulgaris in the serum of healthy relatives (first-degree relatives). However, in the control group (healthy donors) the results were negative. Inheritance of these antibodies in asymptomatic relatives was associated mainly with the histocompatibility complex (human leucocyte antigens - HLA), most often with the DR4 or DR6 haplotypes of the patients. It is likely that the disease appears in susceptible individuals with low antibody levels after a second (external) factor induces the synthesis of high levels of antibody sufficient to cause blisters to form [27]. A condition for the mode of inheritance of these diseases is the distribution of HLA alleles in the population or among affected consanguineous children [28, 29]. After analyzing the distribution of DR4, DQ8 on MHC (major histocompatibility complex) haplotypes among patients with pemphigus vulgaris, it was revealed that the HLA susceptibility gene is dominant [23].
Although there is a genetic predisposition to pemphigus, the question of why relatives of patients do not develop the clinical picture of the disease remains unanswered. Some studies have shown that antibody titers detected in the serum of relatives were not high enough to bind antigens in the skin and cause acantholysis in pemphigus [26].
In almost all of these studies, circulating antibodies were detected by indirect immunofluorescence testing of serum, while direct immunofluorescence testing of clinically intact skin was generally negative [21]. This suggested the presence of immunological or mechanical barriers that prevent the interaction of circulating IgG antibodies with skin antigens. This barrier can be disrupted by drugs, malignancies, viral infections or trauma, which can lead to the development of clinical manifestations of the disease. Studying variability in keratinocyte susceptibility or epidermal permeability may thus help determine who will develop clinical disease that remains asymptomatic despite circulating pemphigus antibodies [25].
Based on the results of these studies [30], we can conclude the following: although immunogenetic factors constitute the main part in the pathogenesis of pemphigus, they are not sufficient for the manifestation and development of the disease. The prevailing idea is that pemphigus occurs due to a combination of endogenous and exogenous factors in people genetically predisposed to the disease [31, 32]. These factors are numerous and include drugs, ultraviolet radiation, stress, infections, hormonal disorders, etc. [33–39].
The role of drugs in the development of autoimmune pemphigus
In many patients with pemphigus, possible additional etiological factors may be found, such as drugs or environmental factors (physical agents, infections) [40]. The role of drugs in the pathogenesis of pemphigus has become known since 1969, when R. Degos et al. [41–45] described a case of pemphigus caused by penicillamine, and a group of thiol substances was identified among other drugs. The thiol group includes penicillamine, which is now rarely used, and drugs that are more commonly used (some angiotensin-converting enzyme inhibitors, such as captopril). In France, these drugs were thought to cause pemphigus-like rashes (pemphigus medicamentalis) in 10% of cases [46]. Drug-induced pemphigus can be divided into two groups: induced pemphigus and trigger pemphigus [42, 44]. With induced pemphigus, the disease regresses after the drug that causes it is discontinued. Trigger pemphigus persists even after the abolition of the provoking factor [47]. It is difficult to make a diagnosis with this syndrome, since not only the clinical picture is similar to that of autoimmune (idiopathic) pemphigus, but also the interval between the first dose of the drug and the onset of manifestations of the disease can take several days or even years [41, 42]. The most characteristic sign of drug causation is the patient's recovery after discontinuation. However, in more than 50% of patients with drug-induced pemphigus, the disease continues to progress despite discontinuation of the drug [48].
The main groups of drugs that can cause pemphigus: thiol drugs - drugs with thiol (captopril, penicillamine, pyritinol, tiopronin) and with thiol metabolites (carbimazole, penicillin, piroxicam); drugs that do not contain thiol - antibiotics (cephalosporins, ethambutol, isoniazid, pentachlorophenol, rifampicin), pyrazole drugs (aminophenazone, aminopyrine, azapropazone, noramidopyrine, phenylbutazone); other drugs - acenocoumarol, heroin, hydanthion, imiquimod, immunomodulators (interleukin-2, interferon-α2, interferon-β), isotretinoin, lysine acetylsalicylate, phenobarbital, progesterone, propranolol, drugs with a sulfhydryl group [41, 42, 44, 45] .
The role of food in autoimmune pemphigus
The thiol group, in addition to drugs, is also part of the molecular structure of substances found in certain foods. That is why, when consuming these products, the same effects can occur as when using drugs containing a thiol group [49]. in vitro studies
It has been shown that three compounds contained in garlic (allimercaptan, allimethyl sulfide, allisulfide) cause acantholysis [50].
Garlic, like onions, shallots and leeks, belongs to the Alliuma
and contains a thiol group.
In the mountainous (Himalayan) zones of India, there are many wild varieties of Allium L.
, which are constantly appearing in the markets [51]. Evidence of the involvement of thiol-containing foods in autoimmunity comes from case reports that indicate the onset of autoimmune pemphigus after consumption of garlic and onions [52, 53]. Removing these foods from the diet contributed to the onset of remission, and the resumption of their consumption caused an exacerbation.
Various thiol groups are also found in the mustard family, which has 3200 species and 375 genera and is ubiquitous [54]. Mustard belongs to the Cruciferae family, as do horseradish, turnips, broccoli, radishes, cabbage, Brussels sprouts and cauliflower. Depending on the chemical structure, the composition of these products may be an irritant, capable of causing antibody-induced acantholysis, or penetrate the epidermis, leading to non-immunological biochemical acantholysis, similar to thiol drugs [55]. Thus, seborrheic pemphigus was induced by allergic contact dermatitis caused by tincture of benzoin gum or contact with chromate; Cases of pemphigus caused by contact with phenols have been described [56, 57]. Tannins are products containing large amounts of biologically active phenols. The astringency of many fruits as they begin to grow is due to their high tannin content, which decreases as the fruit ripens. Polyphenolic compounds induce apoptosis [58]. Tannins also cause the fixation of IgA on erythrocytes and react with structures on the surface and in the erythrocyte membrane [59]. Tannin is often used as a cross-linker [60]. Such cross-linking in the epidermis may be a mechanism through which such compounds are capable of causing toxic pemphigus. Similarly, in drug-induced acantholysis, the first step is to anchor the drug to the cell membrane. Tannins can activate platelets. in vitro studies
tannin had a cytotoxic effect on human peripheral blood lymphocytes. This effect depended on increasing concentration and duration of exposure [61].
Tannin is found in foods such as cassava, or yucca (common in South America, Africa and Asia), mango, guarana, nuts, kola, black walnuts, raspberries, cherries, cranberries, blackberries, rosemary, vanilla, coffee, seeds cocoa, ginger root, ginseng and garlic, tea, mate, various spices such as coriander, cumin, black pepper, garlic [62]. There is evidence that tannin is found in the water of South American rivers, in particular in endemic areas of Brazil. Most patients with endemic pemphigus foliaceus live in close proximity to rivers. Many of these rivers contain large amounts of tannin from surrounding forests. The number of new cases of endemic pemphigus increases during the rainy season and decreases during the dry season. Rainfall causes an increase in water levels in rivers and, accordingly, the amount of organic substances in the water, including tannins, increases. Chlorination of water leads to the formation of hypochlorite ion, which combines with phenol-containing compounds even at high dilutions. In this way, attempts can be made to control the spread of endemic pemphigus [49].
Summarizing the above, we can conclude that diet plays a significant role in the development of autoimmune pemphigus:
1) the molecular structure of substances in many foods is similar to the structure of drugs that can cause pemphigus;
2) cases have been described indicating a connection between the occurrence of the disease and the nature of food [52, 53];
3) tannins and phenols actively interact with proteins;
4) seasonal changes in the incidence of pemphigus in some countries coincide with seasonal changes in the tannin content of the diet;
5) the incidence rate of endemic pemphigus decreases as the population decreases in rural areas and moves to cities with central water supply [49].
Involvement of pesticides in the development of autoimmune pemphigus
The works of a number of authors [63] indicate the role of chemicals, including pesticides and various materials for gardening, in the pathogenesis of autoimmune pemphigus. L. Krain [64] reported a first series study of 14 patients who developed pemphigus after exposure to various chemicals and gardening materials and concluded that pesticides were the most likely cause of the illnesses. After this study, data began to appear in the literature [57, 65] about the occurrence of pemphigus after contact with phosphamides, chromium salts, pentachlorophenol and other agents.
The results of a comparative study conducted in Israel regarding the type of activity of patients indicate a connection between the nature of activity and the occurrence of autoimmune pemphigus. At work, most of the patients studied were exposed to chemicals, most often pesticides used for gardening. In addition, 1/3 of patients at the onset of their illness lived in rural areas, where exposure to pesticides is greater than in the city [39].
There are several mechanisms that could explain the role pesticides play in the pathogenesis of pemphigus. Many of them can cause direct toxic effects on desmosomes, while others activate the immune system, especially T helper cells, which produce many cytokines, which causes increased production of AAT [66]. It has been hypothesized that the acetylcholine receptor and cell surface proteins altered by various pesticides are the target of pemphigus antibodies [67].
The role of ultraviolet radiation in the development of autoimmune pemphigus
Among physical factors, exposure to ultraviolet rays is the most commonly reported. In Israel, studies were conducted that indicated that approximately 40% of patients were continuously exposed to ultraviolet radiation for 5 years before developing pemphigus. Data on the location of lesions in sun-exposed areas and the high prevalence of erythematous (seborrheic) pemphigus in subjects support the results of these studies. However, the question remains whether the mechanism is simply a phototoxic effect or entails a specific immunomodulatory process [39].
Keratinocytes synthesize and secrete the enzyme urokinase-type plasminogen activator (uPA), which binds to specific urokinase-type plasminogen activator receptor (uPAR) matrix ribonucleic acid (mRNA) on keratinocytes [68]. When the receptor binds to uPA, urokinase proteolytically cleaves many extracellular components, leading to pericellular proteolysis. The activity of uPA is controlled by a specific protein called plasminogen activator inhibitor (PAI). Activation of the urokinase system is observed in various physiological and pathological conditions, including secondary epithelialization of skin wounds, oncology and autoimmune skin diseases, including various forms of pemphigus [68–70].
One of the pathophysiological mechanisms, in addition to AAT binding, that contribute to the development of acantholysis and blistering is the activation of proteases, especially uPA. This view is supported by some data [71]: using immunohistological methods, uPA and uPAR were detected in the acantholytic epidermis of patients with pemphigus vulgaris, but were not detected in the skin of healthy individuals. It was also found that in cultured keratinocytes, the IgG fraction from the sera of patients with pemphigus vulgaris induces the expression of uPA and uPAR [72]. Pemphigus or plasminogen-induced acantholysis in skin organ cultures is inhibited by anti-uPA antibodies, anti-uPAR antibodies, purified PAI-2, or synthetic proteinase inhibitors [73–76]. Plasmin is the active enzyme in acantholysis [77].
Enzyme immunoassay studies have shown that ultraviolet B (UVB) at a concentration of 15 mJ/cm2 24 hours after irradiation increases the production of cell-associated uPA and the content of uPA in supernatants, and this phenomenon persists for 48 hours after irradiation. UVB radiation also increases the uPAR concentration in irradiated cell culture extracts by more than 2 times. Also, UVB irradiation increases the amount of uPAR by about 5 times. The mRNA level increases slowly, reaching a maximum value 24 h after irradiation, with no obvious decrease during the remaining observation time (up to 36 h after irradiation) [78].
Thus, in in vitro
UVB has been shown to induce the production of uPA and uPAR.
This suggests that activation of the uPA proteolytic system by ultraviolet radiation may contribute to the photoprovocation of acantholytic lesions in pemphigus. A unique feature of uPA is that when attached to uPAR, its proteolytic activity is concentrated pericellularly, close to desmosomes. Along with in vitro
demonstrating inhibition of antibody-induced acantholysis by urokinase inhibition, the data described above demonstrate uPA as a mediator in the exacerbation of acantholysis by ultraviolet irradiation [73–76, 78].
Based on the data obtained, it was shown that UVB irradiation promotes the expression of two components of the proteolytic system - uPA and uPAR in epidermal keratinocytes [78]. Induction of epidermal components of the urokinase system by UVB radiation may help explain the mechanisms responsible for photosensitivity in pemphigus.
Involvement of viral infection in the development of autoimmune pemphigus
There have been many reports on the possible role of viral infections in the development and/or exacerbation of pemphigus [64, 79, 80–83]. L. Krain [64] first noted the onset of pemphigus after an infection with the herpes simplex virus (HSV). There are also works that describe the onset and/or exacerbation of the disease after an infection caused by the herpes zoster virus, cytomegalovirus and Epstein-Barr virus [80-82]. E. Schlupen et al. [84] reported 3 cases of exacerbation of autoimmune pemphigus caused by HSV, which was detected by polymerase chain reaction (PCR). Treatment with acyclovir resulted in healing of skin lesions. M. Tufano in patients with autoimmune pemphigus also demonstrated the presence of deoxyribonucleic acid (DNA) of HSV types 1 and 2 in 50% of cases in the peripheral blood and in 71% of damaged skin; DNA of the Epstein-Barr virus in 15% of cases is in the peripheral blood and in 5% in damaged skin and DNA of the human herpes virus type 6 in 20% of cases only in the peripheral blood [85]. However, the failure to detect herpesvirus DNA in some patients suggests that viral infection may be incidentally present and detected during the onset and/or exacerbation of autoimmune pemphigus. However, there are many reports demonstrating an association between the onset of autoimmune pemphigus and viral infection, where the onset of pemphigus was preceded by a viral infection [34, 86–88].
The presence of DNA of human herpes virus type 8 (HHV-8) was detected in the affected skin of patients with pemphigus vulgaris and pemphigus foliaceus [89, 90]. These findings suggest that HCH-8 plays a role in more skin lesions than just cutaneous tumors and may be included in the list of factors that play a role in the pathogenesis of pemphigus. In order to assess the extent to which the virus is associated with pemphigus and other bullous dermatoses, a study was conducted that determined the presence of HCH-8 in patients with vulgar and foliate forms of pemphigus, as well as with bullous pemphigoid, acquired epidermolysis bullosa, Dühring's dermatitis herpetiformis, and benign familial pemphigus Gougereau-Hailey-Hailey [91]. When examining skin from the lesion and blood serum using PCR, 160-bp HCH-8 DNA was detected in 7 out of 9 patients with pemphigus vulgaris and 1 out of 2 patients with pemphigus foliaceus. In skin samples from the control group (3 patients with epidermolysis bullosa acquisita, 4 with Dühring's dermatitis herpetiformis, 7 with benign familial pemphigus Gougerot-Hailey-Hailey, 3 patients with HSV detected in the skin, 3 patients with psoriasis and 5 healthy people) ICH -8 not detected. The presence of HCH-8 DNA in the peripheral blood and skin from the lesion of patients with autoimmune pemphigus, but the absence of HCH-8 in clinically intact skin from patients with autoimmune pemphigus and in the skin of patients from the control group suggests that HCH-8 is specific only to damaged skin and is involved only in some moments of the course of autoimmune pemphigus [89, 90].
There may be several explanations for the association between pemphigus and ICH-8. Firstly, HCH-8 can only be an opportunistic infection. Patients with pemphigus usually receive immunosuppressive therapy for a long period, and in some cases of pemphigus virus infection, viral infection appears due to glucocorticoid therapy and may appear before clinical manifestations of pemphigus [34, 86]. This means that the risk of developing a herpes infection may increase with immunosuppressive therapy. However, the patients studied did not receive immunosuppressive therapy before the study, and the results of another study did not depend on its implementation [90]. Secondly, there is the possibility of the existence of positive results without pathogenetic justification. This may indicate the presence of persistent herpes virus or intact viral DNA, similar to pathogenic viruses in affected skin [84]. Thirdly, the virus may act as a trigger only in a certain proportion of patients. Herpesvirus infection can cause tissue destruction by dysregulating and increasing the production of humoral and cellular responses, which can lead to the development of pemphigus or increased antigen presentation in genetically predisposed individuals [85, 90].
Interferon-γ, produced by T cells infected with the herpes virus, induces the production of HLA II on the cell membranes of keratinocytes, forming an immunologically active structural site of pemphigus antigen. During chronic viral infection or its reactivation, a cytokine “switch” of type 1 T helper cells to type 2 T helper cells (Th1→Th2) occurs and interleukins (IL-4 and IL-10) are produced, which further stimulates the response antibodies. Thus, the autoimmune cascade is activated. The appearance of pemphigus is induced through cytotoxic effects and AAT [85, 92].
The role of traditional cosmetics in the development of autoimmune pemphigus
In Tunisia, as in other parts of the Arab world, pemphigus foliaceus predominates. It is more common in young women [93, 94]. A comparative study of the epidemiology of pemphigus in Tunisia and France showed that the incidence of both foliaceous and vulgar forms of pemphigus is higher in Tunisia and is more common in young women from rural areas [95]. However, the lack of incidence among children and the predominance of young women affected contrasts with data obtained in Brazil. A study was conducted to identify risk factors in Tunisia: factors such as pregnancy, use of contraceptives, environmental factors (including in Brazil), traditional attributes of Tunisian life (traditional cosmetics, visiting a Turkish bath) were taken into account [96].
It was noted that almost all sick women used traditional cosmetics (eye kohl, henna). Also, sick people visited Turkish baths more often than healthy people. Tunisian women usually start using traditional cosmetics after marriage for daily care and for social occasions [97]. Traditional eye paint appears to be composed of antimony, and some samples have been found to contain elements such as lead, aluminum, carbon, iron, titanium, silver, and silicon [97, 98]. To obtain henna dye, dry leaves of lawsonia
, which is mixed with oils and various substances such as coal powder, lamp soot and “secret formulas”, possibly containing p-phenylenediamine. Henna often causes reactions such as urticaria, Quincke's edema, and contact dermatitis [99, 100]. It is believed that henna can be a strong allergen.
Thus, the frequent use of traditional Tunisian cosmetics by young women may indicate that traditional cosmetics are a trigger factor for the development of pemphigus. The very high prevalence of cosmetic use compared with the relatively low incidence of pemphigus supports the hypothesis of an interaction between individual susceptibility and risk factors. Thus, in Tunisia, pathogenic AAT reactions in predisposed individuals may be induced and maintained by chronic antigenic stimulation associated with the use of cosmetics [96].
Conclusion
Thus, when studying the pathogenesis of autoimmune pemphigus, a large role is given to the study of pemphigus AAT - IgG and their subclass characteristics in this bullous dermatosis. The pathogenicity of IgG autoantibodies has been confirmed experimentally in animal models. With intraepidermal administration of IgG antibodies to patients with different forms of pemphigus, animals developed bullous rashes similar to those associated with pemphigus.
Of significant interest to researchers is the study of the influence of antibodies of the IgG subclasses on the development of autoimmune pemphigus. Thus, most researchers have noted the presence in the serum of patients with pemphigus of antibodies containing all four subclasses of IgG. However, the most common subclasses were IgG1 and IgG4. Moreover, the level of IgG4 antibodies was significantly higher in patients with pemphigus than in their relatives. This led to the conclusion that IgG4 is the main subclass in autoimmune pemphigus, regardless of the clinical forms of its manifestation.
One of the proofs of the autoimmune nature of pemphigus is a genetic predisposition to it. Numerous studies have shown the connection of the disease with HLA class II, and a diversity of haplotypes has been identified in different races. There is evidence that, regardless of race, the DRB1*04 and DRB1*14 genes are found in pemphigus vulgaris. They are detected in most patients with pemphigus foliaceus. However, despite a genetic predisposition, not everyone develops the disease. It has been shown that autoimmune pemphigus develops in genetically predisposed representatives under the influence of provoking factors. Determining such factors worries scientists in most countries of the world. Many studies have shown the provoking role of medications, food, sun exposure, hormonal disorders, viral infections, and prolonged contact with certain chemicals. There are different opinions regarding the mechanism of action of provoking factors. Some authors believe that such factors can have a direct toxic effect on desmosomes, while others believe that exposure to provoking factors can activate the immune system (by synthesizing many cytokines) and, accordingly, cause increased production of AAT.
Thus, the study of autoimmune pemphigus at the molecular biological level contributes not only to elucidating the pathogenesis of this disease at the present stage, but also expands practical clinical knowledge. The latter allows doctors to predict the course and carry out preventive measures to prevent the development and recurrence of such a life-threatening disease.
Disease prevention
To prevent the development of viral pemphigus, it is recommended to follow simple prevention methods:
- Children at any age should have their own personal hygiene equipment. This is especially true for toothbrushes, washcloths and towels. The towel must be changed every two days. Before this, iron the item at high temperature;
- You should maintain proper nutrition and exclude harmful foods. The diet should contain all the necessary vitamins and minerals to improve immunity;
- The children's room must be kept clean;
- Clothing must be clean and changed daily;
- Avoid contact with infected people;
- Maintain proper hygiene, especially after visiting public places;
- Treat all emerging diseases in a timely manner, do not self-medicate.
Following these simple preventive measures can reduce the likelihood of contracting viral pemphigus. However, parents should be aware that no one is protected from the risk of becoming infected with pemphigus.
2. Reasons
As stated above, the etiopathogenesis of acantholytic pemphigus has not yet been precisely established.
Various hypotheses have been put forward and considered in this regard (viral, metabolic, neurogenic, toxic, hormonal, etc.), but convincing evidence has been obtained only that autoimmune mechanisms play a leading role in the development of pemphigus. However, the root causes of such an attack from one's own immunity, trigger factors and risk factors (with the exception of being female or belonging to a certain genetic line) are still unknown.
Visit our Dermatology page
Complications
If viral pemphigus is not treated correctly, the following types of complications may occur:
- Formation of a chronic form of the disease;
- Infection of wounds with discharge of pus;
- Malfunctions of internal organs;
- Meningitis;
- Development of pneumonia;
- Major weight loss;
- Possibility of spots and scars after treatment of the disease.
Pemphigus can cover a child's entire body and cause other types of skin diseases that make the viral infection worse.