Signs and code according to ICD-10
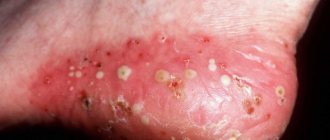
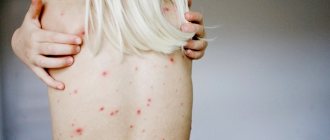
ICD 10 code for suppuration of a postoperative wound is assigned to T81. The clinical picture of damage to the surgical site depends on the causative agent of the pathology. Staphylococci, streptococci, clostridia, Pseudomonas aeruginosa, and pneumococci can get inside damaged skin. Bacteria multiply inside the body. Their active life activity is manifested by local and systemic symptoms.
When a wound becomes infected with gram-positive flora, a person experiences a sharp increase in temperature on the 2nd or 3rd day after surgery. The patient associates the deterioration in general well-being with signs of pain in the damaged area.
During a postoperative medical examination, swelling and hyperemia of the edges of the skin defect with suppuration are detected. Palpation causes unpleasant pain. If the infectious process is localized under the aponeurosis, subcutaneous adipose tissue, symptoms of inflammation appear later. Late detection of the pathological process ends in the development of infectious complications.
Gram-negative flora causes a temperature reaction on the 3-4 postoperative day. Intoxication, pain syndromes are more pronounced than when the wound is infected with gram-positive bacteria. The patient shows signs of involvement of the cardiovascular system in the pathological process. He suffers from palpitations, tachycardia, pressure changes, and cardialgia.
Non-clostridial anaerobic microflora causes systemic and local manifestations of suppuration from the first days. The patient experiences high body temperature, severe pain in the area of the fresh postoperative wound, weakness, and decreased appetite. When examining the surgical site, pronounced swelling and hyperemia of the pathological area of the body is determined. Based on these signs, infection with bacterial agents can be suspected.
2-3 days after the development of suppuration, secretion begins to be released from the wound. With a staphylococcal infection, it has a creamy consistency and a yellow-green color. There is no unpleasant odor coming from the postoperative area.
A sign of the activity of Pseudomonas aeruginosa is manifested by the formation of a blue-green secretion with a liquid consistency. A specific smell and characteristic discharge from the wound help to suspect infection with this pathogen.
The diagnosis of suppuration of the postoperative area is established on the basis of clinical signs of the disease, medical history, provoking factors, and the results of diagnostic studies.
Treatment of long-term non-healing postoperative wounds of the perineum and anal canal
According to the definition of a special meeting of the European Society for Tissue Repair, “a wound should be considered chronic if it does not heal within a period that is normal for wounds of this type or location” [7]. According to our data, a wound process that exists for more than 45 days without signs of active healing is considered chronic.
Recently, the number of patients infected with sexually transmitted microorganisms continues to grow steadily. Sexually transmitted diseases (STDs) are often asymptomatic or minimally symptomatic, so many patients do not consult a doctor; in this regard, sexually transmitted infections have a strong tendency to become chronic [2].
In perineal wounds after surgical interventions, there are polymicrobial associations represented by opportunistic bacteria [3], including clinically significant ones, in combination with chlamydia, trichomonas, gardnerella, mycoplasma, ureaplasma, cytomegalovirus (CMV), genital herpes (virus herpes simplex type 2 (HSV-2)), as well as candida, i.e., sexually transmitted pathogens [1, 4, 8, 11, 12].
However, there are very few works devoted to this problem; they mainly concern gynecological and urological practice. In this regard, to study the influence of sexually transmitted infections on the course of the wound process, we decided to study the microbiocenosis of postoperative wounds in patients after operations on the distal rectum, anal canal and perineum.
A study was conducted, the purpose of which was to study wound microbiocenosis and develop a complex of specific corrective therapy in the postoperative period, aimed at improving the results of treatment of patients with long-term non-healing wounds of the anal canal and perineum.
Materials and methods
The study has been conducted from September 2010 to the present. The study included 96 people who underwent surgery at the State Scientific Center of Coloproctology for rectal fistulas, chronic anal fissures and hemorrhoids.
The main group consisted of 49 patients with a sluggish wound process, confirmed by clinical cytological examination, which resulted in long-term non-healing wounds (46 to 118 days passed from the day of surgery, on average 81.3 ± 49.3). The control group consisted of 47 patients, in whom, according to clinical and cytological studies, no deviations in the course of the wound process were noted; the wounds healed in normal periods - on average 37±10.5 days (p<0.05).
A comparative analysis of 2 groups of patients did not reveal significant differences in gender and age composition. Thus, in the main and control groups there were more men - 28 (57.1%) and 29 (61.7%), respectively. There were 21 (42.9%) women in the main group, and 18 (38.3%) in the control group.
The age of the patients in the main group ranged from 19 to 74 years, but most of them were people of working age, their average age was 41.0±13.2 years. In patients in the control group (persons from 18 to 72 years old), the average age was 42.8±12.2 years.
The patients were also comparable in terms of nosological forms. Among the 49 patients of the main group, there were 13 (26.5%) patients with chronic anal fissures, 28 (57.1%) with rectal fistulas and 8 (16.3%) with hemorrhoids. The majority of patients in the control group - 24 (51.1%) were also diagnosed with fistulas, 13 (27.7%) had fissures and 10 (21.3%) had hemorrhoids.
There were also no significant differences in the nature of surgical interventions. In most cases, patients in both the main and control groups underwent surgical interventions involving excision of fistulas into the lumen of the rectum: in 21 (42.9%) and 17 (36.2%), respectively. Excision of fistulas with sphincter suturing was performed in 3 (6.1%) patients in the main group and in 5 (10.6%) patients in the control group. Excision of the fistula with reduction of the flap was performed in 4 (8.2%) patients in the main group and 2 (4.3%) in the control group.
21 (42.8%) (11 men and 10 women) patients in the main group had indications of a history of STDs and diseases that could be caused by sexually transmitted infections, while patients in the control group had no indications of such diseases. in one observation (p<0.001). The following diseases were identified in the patients of the main group: 2 (4.1%) were observed by a urologist for chronic prostatitis; 2 (4.1%) patients were diagnosed with colpitis in the past, but the causative agents of this disease were not identified; indication of previous adnexitis occurred in 1 (2.0%) patient; 1 patient (2.0%) was treated for Reiter's syndrome; 5 men (10.2%) suffered from trichomoniasis; 3 patients (6.1%) (a woman and two men) – chlamydia; 2 patients (4.1%) had gardnerellosis and 3 (6.1%) had candidiasis, as well as 1 (2.0%) had genital herpes; 1 patient (2.0%) had ureaplasmosis in the past (Table 1).
In all patients in the postoperative period, bacteriological and bacterioscopic examinations of wound discharge from the anal canal and urethra were carried out; in women, additionally from the cervical canal: in patients of the control group - on the 25th day after surgery and in patients of the main group - at the time of treatment, and then 14 days after the start of treatment.
Using a light microscope, the causative agents of infections such as trichomoniasis, candidiasis, gardnerellosis, and the presence of gram-positive and gram-negative cocci and rods were determined. In addition, the polymerase chain reaction (PCR) method was used to diagnose sexually transmitted infections. In all patients in whom chlamydia, mycoplasma, ureaplasma, and gardnerella were detected, the diagnosis was confirmed by direct immunofluorescence. When HSV-2 and CMV were detected, the diagnosis was confirmed using the culture method. Microbiological studies were carried out in a bacteriological laboratory using a technique that allows us to determine the quantitative content and species spectrum of aerobic and facultative anaerobic microorganisms.
In patients of the main group, immediately after treatment for a long-term non-healing wound (on the 46th–118th day), and then 14 days after the start of treatment, cytological and pathomorphological studies of discharge from postoperative wounds were carried out. In patients in the control group, these studies were carried out on the 25th day after surgery.
results
During a cytological examination during treatment for a long-term non-healing wound, 45 (91.8%) patients of the main group showed a picture of “perverted” repair - the presence of multinucleated cells such as foreign bodies in smears from wounds, and also in all 49 patients - chronic inflammatory process with a large number of unchanged leukocytes. The number of leukocytes in wounds from 30 to 40 per field of view was observed in 8 (16.3%) patients, 40–60 in 7 (14.3%), 60–80 in 9 (18.4%), the entire field vision – in 25 (51%) patients. By the 25th day, in all patients in the control group, leukocytes with signs of dystrophy were in small numbers and did not exceed 5 per field of view (p <0.001).
The cytological examination data correlated with the data obtained during the pathomorphological examination. In patients of the main group, a large number of unchanged neutrophils were found in the biopsy specimen; the microflora is localized both extra- and intracellularly; the phenomena of phagocytosis are weakly expressed. In patients in the control group, the biopsy specimen revealed single, dystrophically altered leukocytes, as well as macrophages and fibroblasts.
During microbiological examination at the time of treatment (46–118 days after surgery), various microorganisms were identified in the discharge of postoperative wounds in all 49 (100%) patients of the main group; In patients in the control group, on the 25th day after surgery, in 44 (93.6%) of 47 observations, any microorganisms were detected, and in 3 (6.4%) of them there was no growth in bacterial cultures.
In patients of the main group, Enterococcus faecalis (fecal streptococci) predominated among microorganisms - in 28 (57.1%), as well as Escherichia coli (Escherichia coli) - in 20 (40.8%); the former occurred more than 4 times more often, the latter – 6 times more often than in patients in the control group. Enterobacter cloacae (Enterobacter) was found 11 times more often in patients of the main group compared to patients in the control group (this microorganism was found in only 1 patient in the control group). Candida albicans (yeast fungus) was cultured in 14 (28.6%) patients in the main group and in 9 (19.1%) in the control group.
In patients of the main group, in bacterial cultures from postoperative wounds such clinically significant microorganisms as Proteus mirabilis (Proteus) were found in 10 (20.4%), Acinetobacter lwoffii (Acinetobacter) - in 4 (8.2%), Staphylococcus aureus (Staphylococcus aureus) – in 10 (20.4%), Streptococcus haemolyticus (hemolytic streptococcus) – in 3 (6.1%) observations. The total frequency of detection of these clinically significant opportunistic microorganisms in patients of the main group was 27 (55.1%) cases, the number of microorganisms exceeded 105 CFU. In patients in the control group, clinically significant microorganisms were detected only in 1 (2.1%) patient (Staphylococcus aureus was cultured); the microbial number in this case was 105 CFU (p <0.001).
Associations of microorganisms in bacterial cultures were found in 47 (95.9%) patients of the main group: 5 different pathogens were found in 5 (10.2%), 4 in 5 (10.2%), 3 in 5 (10.2 %), 2 – in 32 (65.3%) patients; whereas in the control group, only 4 (8.5%) patients had an association of 2 microorganisms (p <0.001) (Table 2).
In all patients of the main group with long-term non-healing wounds, various sexually transmitted microorganisms were identified, of which pathogenic STD pathogens were found in 98% of patients, while in the control group this figure was only 23.4% (in 11 out of 47 patients), Moreover, all detected microorganisms that cause STDs in the latter were opportunistic (p <0.01) (Fig. 1).
In 26 (53.1%), i.e. more than half of the patients in the main group, chlamydia was detected in the wound discharge. At the same time, Chlamydia trachomatis was also found in the urethra and cervical canal of women in only 3 (6.1%) patients. In the control group, chlamydia was not diagnosed (p<0.001).
Trichomonas was detected in more than a third of patients of the main group - in 19 (38.8%) cases: in 2 (4.1%) patients in discharge from wounds, as well as the urethra, anal and cervical canals, and in 2 (4.1%) %) of men - in the urethra and discharge from wounds, and in 15 (30.6%) patients - only in discharge from postoperative wounds. Trichomoniasis was not detected in the control group (p<0.001).
Gardnerella was detected in 17 (34.7%) patients of the main group: in 8 (16.3%) of them this microorganism was found in all the studied material, in 9 (18.4%) - only in the wound discharge. In 7 (14.9%) patients in the control group, this microorganism was detected in 5 (10.6%) observations in the entire study material, in 2 (4.3%) - only in discharge from the urethra and cervical canal.
Candida was detected in 29 (59.2%) cases in patients of the main group in discharge from the anal canal, urethra, cervical canal and postoperative wounds. In the control group, candidiasis was found in 5 (10.6%) people also in the entire study material (p <0.001).
Somewhat less common were intracellular microorganisms such as ureaplasma and mycoplasma. In 6 (12.2%) patients of the main group, ureaplasma was detected in all the studied material, while in the control group this microorganism was detected in 1 (2.1%) patient only in the discharge from the urethra (p <0.05). In 1 (2.0%) case, a patient in the main group was diagnosed with Mycoplasma hominis, which was detected in the discharge of postoperative wounds and the anal canal. In 1 (2.1%) patient in the control group, Mycoplasma hominis was found in the discharge of the postoperative wound and urethra.
In 6 (12.2%) patients of the main group, HSV-2 was detected: in 3 (6.1%) in material from the urethra, cervical canal and postoperative wounds and in 3 (6.1%) only in discharge from wounds. HSV-2 was not found in the control group (p<0.05).
CMV was detected in 10 (20.4%) patients of the main group: in 9 (18.4%) in the anal canal and wound discharge and in 1 (2.0%) patient in the urethra, cervical canal and wound discharge; whereas in the control group it was not detected in any observation (p <0.001).
The frequency of detection of Trichomonas in patients of the main group only from discharge from postoperative wounds in the absence of them in discharge from the genitals was 30.6% (in 15 observations), chlamydia - 46.9% (in 23 observations), CMV - 18.4% ( in 9 observations) and HSV-2 – 6.1%. This fact indicates a long-term chronic process, in which it can be difficult to detect STD pathogens if there is no provoking factor. Since the causative agents of chronic sexually transmitted infections are in the epithelial cell in an inactive, “dormant” state, the appearance of a wound in the perineal area or anal canal is the very provoking factor that allows the chronic latent infection to become active.
STD pathogens in all 49 patients of the main group in whom they were diagnosed were not found as a monoinfection in any observation, but were found only in association with other STD pathogens. The presence of 2 microorganisms causing STDs was found in 33 (67.3%) patients, and in 16 (32.7%) patients of the main group a combination of 3 pathogens was found.
In 11 patients in the control group in whom sexually transmitted microorganisms were identified, on the contrary, 1 pathogen was most common - in 8 (17.0%), a combination of 2 microorganisms was detected in 3 (6.4%) patients, and the presence of 3 or more microorganisms at once was not detected in any observation (p <0.001).
In patients of the main group, the frequency of detection of associations of microorganisms was: Trichomonas, CMV and Candida - 10.2% (5 patients); chlamydia, trichomonas and candida – 8.2% (4 patients); trichomonas, gardnerella and candida – 10.2% (5 patients); chlamydia and gardnerella – 20.4% (10 observations); chlamydia and candida – 14.3% (7 patients); trichomonas and candida – 8.2% (4 cases). In 3 (6.4%) patients in the control group, Candida was found in combination with Gardnerella.
Among the associations of opportunistic microorganisms with STD pathogens in patients of the main group, there were combinations: Staphylococcus aureus with Trichomonas, chlamydia and candida - in 4 (8.2%) patients; Protea with Trichomonas and Candida – in 3 (6.1%); Proteus with chlamydia and candida – in 6 (12.2%). As already indicated, Staphylococcus aureus was detected in culture only in 1 (2.1%) patient in the control group and was associated with candida.
Hemolytic streptococci were associated with enterococci, chlamydia, candida and HSV-2 in 2 (4.1%) patients, in association with chlamydia and candida - in 1 (2.0%) patient of the main group.
Thus, opportunistic, clinically significant microorganisms are often associated with pathogenic microorganisms, which include Trichomonas, Chlamydia, CMV and HSV-2. It can be said that sexually transmitted pathogenic microorganisms create favorable conditions for the proliferation of opportunistic microorganisms, such as Staphylococcus aureus, Proteus, hemolytic streptococcus, etc., which in turn leads to long-term maintenance of the inflammatory wound reaction and slowdown of repair processes in wounds
All patients with long-term non-healing wounds in whom STDs were detected were prescribed appropriate therapy depending on the pathogen isolated.
For chlamydia, antibiotics were prescribed, taking into account that the anti-chlamydial agent should actively act on dividing reticular bodies. These antibiotics include: macrolides (roxithromycin, clarithromycin, josamycin), tetracyclines (tetracycline, doxycycline), erythromycin, fluoroquinolones (ciprofloxacin, ofloxacin).
For mycoplasmosis and ureaplasmosis, patients received antibiotics from the following groups: macrolides (roxithromycin, clatrithromycin, josamycin), tetracyclines (tetracycline, doxycycline), fluoroquinolones (ciprofloxacin, ofloxacin), lincosamines (lincomycin, clindamycin), aminoglycosides (gentamicin, streptomycin).
For trichomoniasis and gardnerellosis, drugs with antiprotozoal and antimicrobial effects were used: metronidazole, tinidazole, ornidazole.
Treatment of candidiasis was carried out with antimycotic drugs from the groups of triazole (fluconazole, itraconazole), imidazole, polyene (nystatin, levorin, amphotericin B), allylamine, echinocandin (caspofungin).
For genital herpes, interferon and compounds with interferon-inducing activity were prescribed; inhibitors of viral nucleic acid replication (nucleoside analogs (acyclovir), pyrophosphate analogs (foscarnet sodium)).
For CMV infection, inhibitors of viral nucleic acid replication were prescribed: ganciclovir, pyrophosphate analogues (foscarnet sodium), potato shoot extract.
All patients were prescribed probiotics in combination with etiotropic drugs to restore normal flora of the gastrointestinal tract.
As a result of the treatment, recovery was noted in all patients with long-term non-healing wounds included in the main group, which was confirmed by clinical, cytological and pathomorphological studies.
Clinical example: patient V., operated on for a transsphincteric fistula of the rectum. She applied for a long-term non-healing wound on the 56th day after surgery. In the wound area, hypergranulations were detected, which bled easily upon contact; in some areas the wound was covered with fibrin (Fig. 2).
PCR and bacterioscopic examination of discharge from the wound revealed trichomonas, chlamydia and candida. After 3 weeks After specific therapy aimed at eliminating identified infectious agents, the wound healed completely.
Clinical example: patient K., operated on for an extrasphincteric fistula of the rectum. On the 108th day after surgery, upon clinical examination, there was copious discharge from the wound of a mucopurulent and mucous-sacral nature (Fig. 3).
Clinical examination data correlated with cytological (Fig. 4) and pathomorphological (Fig. 5) studies. PCR and bacterioscopic examination of discharge from the wound revealed CMV, HSV-2 and candida. After etiotropic treatment (antiviral therapy, antimycotic drugs), the wound healed completely.
Conclusion
1. In patients with a history of long-term non-healing postoperative wounds, the frequency of detected genitourinary infections and diseases possibly caused by STDs was 42.8%, while in patients in the control group there were no anamnestic indications of previous STDs (p <0.001).
2. During cytological examination, smears from wounds showed a picture of perverted repair in 91.8% of patients in the main group. In all patients in the control group, by the 25th day, leukocytes were found only in small quantities (up to 5 per field of view), with signs of dystrophy (p <0.001).
3. The frequency of detection of such clinically significant microorganisms as Proteus, hemolytic streptococci, Staphylococcus aureus, Acinetobacter in patients of the main group was 55.1%. And only 1 (2.1%) patient in the control group was cultured with Staphylococcus aureus (p <0.001). Associations of microorganisms in bacterial cultures were found in 95.9% of patients in the main group, while in the control group - only in 8.5% of patients (p <0.001).
4. All patients of the main group with long-term non-healing wounds had various STDs, including obligate pathogenic microorganisms in 98%. In 23.4% of patients in the control group, the detected sexually transmitted microorganisms were only opportunistic (p <0.01).
5. In the majority of patients with long-term non-healing wounds, trichomonas (30.6%), chlamydia (46.9%) and CMV (18.4%), herpes viruses (6.1%) were detected only in the discharge from the wounds and were not detected in scrapings from the genitals. The presence of a wound defect in the perineal or anal canal is a provoking factor that allows a chronic, latent infection to become active.
6. Clinically significant opportunistic pathogens (Proteus, Acinetobacter, hemolytic streptococcus, and Staphylococcus aureus) are most often associated with sexually transmitted pathogens, which include Trichomonas, Chlamydia, CMV, and HSV-2.
Reasons for the appearance of an abscess on a postoperative suture
In traumatological, orthopedic, and surgical medical practices, it is generally accepted that any wound is considered infected. Inside damaged skin there is a certain amount of bacteria. Signs of suppuration may not appear. For infection of the postoperative area to develop, the presence of one or a combination of provoking factors must be present:
- large depth of defect;
- the presence of foreign bodies and blood in the wound;
- a sufficient number of bacteria (staphylococci, streptococci, E. coli, salmonella, shigella);
- the presence of necrotic tissue in the postoperative area.
The reasons for the development of suppuration are separately identified:
- The appearance of signs of an abscess is associated with non-compliance with the rules of asepsis and sterilization by medical workers. Poorly processed instruments and insufficiently washed surgeon's hands transfer a huge amount of bacteria into the wound. After removal of the pathological focus, some microorganisms remain. They begin to actively multiply, which leads to the appearance of typical symptoms of suppuration in the postoperative area. Often signs are observed after cesarean section or appendectomy.
- A pathological process in a wound can develop if the patient is hypersensitive to surgical materials. In some cases, a reaction of rejection of implants, prostheses, dressings and suture elements occurs. Signs of suppuration appear a couple of days after surgery.
- A high risk of developing an abscess in the postoperative area is observed in people with endocrine diseases. These include diabetes mellitus.
- Signs of wound suppuration appear against the background of a weakened immune system. The body cannot fight the pathogenic bacteria that surround a person. Microorganisms easily penetrate deep into the tissue defect and cause typical symptoms.
- The pathological process develops when harmful microorganisms are transferred from an infected lesion to healthy tissue. During an operation to remove appendicitis, paraproctitis, lung lobes, fallopian tubes, and hemorrhoids, some bacteria are introduced into the area of the tissue incision. In the postoperative wound, suppuration begins.
- Improper application of a ligature suture can cause signs of a purulent process.
The pathological condition requires identification of the etiological factor in order to prescribe adequate therapy.
Complications of wounds. Basic principles of treatment
Wounds can be accompanied by a variety of complications, both immediately after the wounds are inflicted and in the long term. Wound complications include:
· The development of traumatic or hemorrhagic shock is the earliest and most serious complication. In the absence of immediate help, it causes an unfavorable outcome.
· Seromas are accumulations of wound exudate in wound cavities, dangerous due to the possibility of suppuration. When seroma develops, it is necessary to ensure the evacuation of fluid from the wound.
· Wound hematomas are formed due to incomplete stopping of bleeding. Hematomas are potential foci of infection; in addition, by squeezing surrounding tissues, they lead to ischemia. They must be removed by puncture or during wound revision.
· Necrosis of surrounding tissues - develops when the blood supply to the corresponding area is disrupted due to tissue trauma during surgery or improper suturing. Wet skin necrosis must be removed due to the risk of deep accumulations of pus. Superficial dry necroses of the skin are not removed, as they perform a protective function.
· Wound infection - its development is facilitated by a high level of contamination and high virulence of the microflora that has entered the wound, the presence of foreign bodies in the wound, necrosis, accumulations of fluid or blood, damage due to injury to bones, nerves, blood vessels, chronic disruption of local blood supply, as well as late surgical treatment and general factors influencing the course of the wound process. Experimental and clinical studies have established that in most cases, for the development of an infectious process in a wound, it is necessary for its contamination to exceed a critical level of 105-106 microorganisms per 1 gram of tissue. Among the general factors contributing to the development of wound infection, significant blood loss, the development of traumatic shock, previous fasting, vitamin deficiencies, overwork, the presence of diabetes mellitus and some other chronic diseases play an important role.
The development of pyogenic infection is caused by staphylococcus, Pseudomonas aeruginosa, Escherichia coli and other pyogenic bacteria, anaerobic infection - by clostridia and non-clostridial anaerobic microflora, erysipelas - by streptococci. When a wound infection generalizes, sepsis develops. Most often, the development of pyogenic wound infection occurs on days 3-5 after injury, less often at a later date - on days 13-15. Anaerobic infection can develop very quickly; in fulminant forms, it is diagnosed a few hours after injury.
If it gets into the wound with soil, dust, foreign bodies, Cl. Tetani may develop tetanus. In the absence of specific prevention, the probability of contracting tetanus in the presence of contaminated wounds reaches 0.8%. The rabies virus can enter the body through bite wounds.
· Dehiscence of wound edges - occurs in the presence of local or general factors that impede healing, as well as when sutures are removed too early. During laparotomy, the divergence of the edges of the wound can be complete - with eventration, that is, with internal organs coming out, incomplete - with preservation of the integrity of the peritoneum, and hidden, when the integrity of the skin is preserved. Dehiscence of the wound edges is eliminated surgically.
· Complications of scars are the formation of hypertrophied scars and keloids. Hypertrophied scars develop when there is a tendency to excessive formation of scar tissue and most often when the wound is located perpendicular to the Langer line. Keloids, unlike hypertrophied scars, have a special structure and spread beyond the boundaries of the wound. Complications of scars lead not only to cosmetic, but also to functional defects, such as impaired walking or upper limb function due to limited range of motion in the joints. Surgical correction is indicated for hypertrophied scars with impaired function, but for keloids it often leads to a deterioration in the treatment outcome.
· Long-term chronic wounds can be complicated by the development of malignancy. The diagnosis is confirmed by a biopsy of wound tissue. Surgical treatment requires radical excision within healthy tissue.
Basic principles of wound care
Treatment for injuries usually takes place in two stages - the first aid stage and the qualified assistance stage.
¨ First aid stage
When providing first aid at the site of injury, two main tasks are solved: stopping bleeding and preventing further microbial contamination. First aid includes the use of available methods to temporarily stop bleeding, pain relief, application of a protective bandage and transport immobilization. At this stage, you should not wash the wound or remove foreign bodies from it.
¨ Qualified assistance stage
At the stage of hospital care, the following tasks are solved:
· prevention and treatment of wound complications;
· acceleration of the healing process;
· restoration of functions of damaged organs and tissues.
Basic principles of wound treatment:
· strict adherence to asepsis at all stages of treatment;
· mandatory surgical treatment;
· active drainage;
· the earliest possible closure of wounds with primary or secondary sutures or using autodermoplasty;
· targeted antibacterial and immunotherapy, correction of systemic disorders.
To select adequate wound treatment tactics, a thorough assessment of its condition is necessary, and the following is assessed:
· Localization, size, depth of the wound, damage to underlying structures such as fascia, muscles, tendons, bones.
· The condition of the edges, walls and bottom of the wound, as well as surrounding tissues, the presence and characteristics of necrotic tissue.
· The quantity and quality of exudate - serous, hemorrhagic, purulent.
· Level of microbial contamination. The critical level is the value of 105 - 106 microbial bodies per 1 g of tissue, at which the development of a wound infection is predicted.
· Time elapsed since injury.
¨ Treatment of contaminated wounds
The risk of developing wound complications in the presence of contaminated wounds is much higher than in aseptic wounds. Treatment of contaminated wounds consists of the following steps:
· In case of possible contact of the wound with the ground (all injuries with a violation of the integrity of the body, frostbite, burns, gangrene and tissue necrosis, out-of-hospital births and abortions, animal bites), measures are necessary to prevent a specific infection - tetanus, and in case of animal bites - rabies.
In order to prevent tetanus, vaccinated patients are administered 0.5 ml of adsorbed tetanus toxoid, unvaccinated patients - 1 ml of toxoid and 3000 IU of tetanus toxoid serum. Due to the danger of developing anaphylactic reactions to protein, the administration of anti-tetanus serum is carried out according to Bezredko: first, 0.1 ml of diluted serum is injected intradermally, if the size of the papule is less than 10 mm, after 20 minutes 0.1 ml of undiluted serum is injected subcutaneously, and only if there is no reaction to subcutaneous administration After 30 minutes, the entire dose is administered subcutaneously.
In case of bites from animals (dogs, foxes, wolves, etc.) suspected of rabies, or their saliva getting on damaged tissue, primary surgical treatment of the wound cannot be performed. The wound is only washed and treated with an antiseptic. There are no stitches. A course of subcutaneous administration of rabies vaccine is required, which is performed in specialized rabies centers, and tetanus prophylaxis. In the presence of superficial injuries (abrasions, scratches) of any location except the head, neck, hands, toes and genitals caused by domestic animals, culture purified concentrated rabies vaccine (COCAV) is administered 1 ml immediately, as well as on 3, 7, 14, 30 and 90 days. But if, when observing the animal, it remains healthy for 10 days, then treatment is stopped after 3 injections.
If animal saliva gets on the mucous membranes, if bites are localized in the head, neck, hands, toes and genitals, as well as with deep and multiple bites and any bites of wild animals, in addition to the administration of COCAB, immediate administration of rabies immunoglobulin (RAI) is necessary. Heterologous AIH is prescribed at a dose of 40 IU per kg of body weight, homologous - at a dose of 20 IU per kg of body weight. Most of the dose should be infiltrated into the tissue surrounding the wound, the rest is administered intramuscularly. If observation of the animal is possible, and it remains healthy for 10 days, then the administration of COCAV is stopped after the 3rd injection.
· In all cases of contaminated wounds, except for minor superficial injuries and cases where there are cosmetic and functional contraindications, primary surgical treatment (PST) with wound dissection, revision of the wound canal, excision of the edges, walls and bottom of the wound is mandatory. The purpose of PSO is the complete removal of non-viable and contaminated tissue. The later PST is performed, the lower the likelihood of preventing infectious wound complications.
PSO is not performed when wounds are localized on the face, as it leads to an increase in cosmetic defect, and good blood supply to this area ensures a low risk of suppuration and active wound healing. With extensive wounds of the scalp, performing PSO in full can lead to the impossibility of matching the edges and closing the wound. Non-penetrating puncture wounds without damage to large vessels and bite wounds with suspected penetration of the rabies virus are also not subject to PSO. PSO can be completed with the application of primary sutures - with suturing tightly or, in the presence of risk factors for wound suppuration, - with drainage left in place.
Preferably, flow-wash drainage of sutured wounds followed by dialysis with effective antiseptics. Flow-flushing drainage is carried out by installing counter perforated drains, one of which is used to administer the drug, and the other is used for outflow. The administration of drugs can be jet and drip, fractional or continuous. In this case, outflow can be carried out in a passive or active way - using vacuum.
This method protects wounds from secondary contamination, promotes more complete removal of discharge, creates conditions for a controlled abacterial environment and favorable conditions for wound healing. When draining, several general principles must be followed. Drainage is installed in sloping areas of the wound cavity, where fluid accumulation is maximum. Removing the drainage tube through the counter-aperture is preferable than through the wound, since the drainage, being a foreign body, interferes with the normal healing of the wound and contributes to its suppuration.
If there is a high risk of developing wound suppuration, for example, in the presence of sudden changes in the surrounding tissues, the application of primary delayed sutures, including provisional ones, is indicated. Like the primary ones, these sutures are placed on the wound before the development of granulation tissue, usually 1-5 days after PSO when the inflammatory process subsides. The healing of such wounds proceeds according to the type of primary intention. Sutures are not applied only after treatment of gunshot wounds and if it is impossible to compare the edges of the wound without tension; in the latter cases, the earliest possible closure of the wound defect using reconstructive surgery is indicated.
· Antibiotic prophylaxis is carried out according to the same scheme as for “dirty” surgical interventions. A 5-7 day course of antibiotics is required.
· Antiseptic prophylaxis involves the use of effective antiseptics at all stages of the operation and when caring for the wound. When treating wounds, chlorhexidine, sodium hypochlorite, dioxidine, lavasept, hydrogen peroxide, potassium permanganate and other antiseptics can be used. Drugs such as furatsilin, rivanol, chloramine are currently not recommended for use in surgical departments, since hospital microflora are resistant to them almost everywhere.
· Wound management after PSO with suturing is similar to the management of surgical wounds. Regular changes of aseptic dressings and drainage care are performed. Treatment of open wounds after PSO is carried out, like the treatment of purulent wounds, in accordance with the phases of the wound process.
¨ Treatment of purulent wounds
Treatment of purulent wounds is complex - surgical and conservative.
· In all cases of infected wounds, when there are no special functional contraindications, secondary surgical debridement (SDT) is performed. It consists of opening a purulent focus and leaks, evacuation of pus, excision of non-viable tissue and mandatory provision of adequate drainage of the wound. If the wound is not sutured after VChO, secondary sutures may be applied in the future. In some cases, with radical excision of an abscess during VCO, primary sutures can be applied with mandatory drainage of the wound. Preferably flow-through drainage. If there are contraindications to carrying out VChO, they are limited to measures to ensure adequate evacuation of exudate.
· Further local treatment of purulent wounds depends on the phase of the wound process.
In the inflammation phase, the main goals of treatment are fighting infection, adequate drainage, accelerating the process of wound cleansing, and reducing systemic manifestations of the inflammatory reaction. The basis is treatment with bandages. For all wounds that heal by secondary intention, wet debridement is considered the standard treatment method. Dry debridement with the application of dry sterile wipes to the wound is used only for temporary covering of wounds and treatment of wounds that heal by primary intention.
Wet treatment uses bandages that create a moist environment in the wound. Osmotically active substances, antiseptics, and water-soluble ointments are used. Fat-soluble ointments are contraindicated as they interfere with the outflow of secretions. It is possible to use modern atraumatic dressings with high absorption capacity that maintain a certain level of moisture and help remove exudate from the wound and firmly retain it in the dressing. Modern combination preparations for local treatment of wounds contain immobilized enzymes - gentacycol, lysosorb, dalcex-trypsin.
Dressings should be changed with adequate pain relief. The frequency of changing dressings depends on the condition of the wound. Typically, 1-2 changes of dressings per day are required, hydroactive dressings such as Hydrosorb can remain on the wound for several days, the need to immediately change the dressing arises in the following cases: the patient complains of pain, a fever has developed, the dressing is wet or dirty, or its fixation is impaired. At each dressing, the wound is cleaned of pus and sequestration, necrosis is excised and washed with antiseptics. Chlorhexidine, sodium hypochlorite, dioxidine, lavacept, hydrogen peroxide, and ozonated solutions can be used to wash the wound. To accelerate necrolysis, proteolytic enzymes, ultrasonic cavitation, vacuum wound treatment, and pulsating jet treatment are used. Physiotherapeutic procedures include ultraviolet irradiation of the wound, electro- and phonophoresis with antibacterial and analgesic substances.
In the regeneration phase, the main goals of treatment are to continue the fight against infection, protect granulation tissue and stimulate repair processes. There is no longer any need for drainage. Dressings applied during the regeneration phase should protect the wound from trauma and infection, not stick to the wound and regulate the humidity of the wound environment, preventing both drying and excess moisture. Dressings with fat-soluble antibacterial ointments, stimulating substances, and modern atraumatic dressings are used.
After complete cleansing of the wound, secondary sutures or adhesive tape are indicated; for large defects, autodermoplasty is indicated. Unlike primary sutures, secondary sutures are applied to granulating wounds after the elimination of the inflammatory process. The goal is to reduce the volume of the wound defect and the entry point for infection. After 21 days, secondary sutures are applied only after excision of the formed scar tissue. In cases where it is impossible to compare the edges to close the defect, autodermoplasty is performed as early as possible - immediately after the inflammatory process has subsided.
In the phase of scar reorganization, the main goal of treatment is to accelerate epithelization and protect the wound from trauma. Since drying causes a crust to form, which slows down epithelization, and epithelial cells die with excess moisture, dressings should still keep the wound in a moderately moist state and protect against injury. Bandages with indifferent and stimulating ointments are applied. Sometimes physiotherapy is used - ultraviolet irradiation, laser, pulsating magnetic field.
· General treatment of purulent wounds includes antibacterial therapy, detoxification, immunotherapy, and symptomatic treatment.
Antibacterial therapy is used in phases 1-2 of the wound process. The drug must be prescribed taking into account the sensitivity of the wound microflora. Systemic administration of antibiotics is indicated; topical administration is not currently recommended. The initial empirical choice of antibacterial therapy, pending sensitivity results, should be directed against typical pathogens, which are staphylococci, streptococci and gram-negative aerobic bacteria.
Amoxiclav, levofloxacin are used, as a reserve - cefuroxime, ciprofloxacin, ofloxacin, and for bites - doxycycline. Treatment of staphylococcal wound infections with pathogen resistance requires the use of vancomycin or linezolid. For erysipelas, penicillins, azithromycin, and lincosomide are indicated. If the infection is caused by Pseudomonas aeruginosa, the drugs of choice are carbenicillin, tazocin, timentin, as well as 3rd generation cephalosporins and fluoroquinolones. In addition to antibiotics, bacteriophages are used in the treatment of purulent wounds.
Detoxification is used in the presence of systemic manifestations of the inflammatory process. Infusions of saline solutions, detoxifying solutions, forced diuresis, and in severe cases, extracorporeal detoxification are used.
Immunocorrective therapy can be specific (vaccines, serums, toxoids) and nonspecific. Tetanus toxoid, antitetanus and antigangrenous serum, antitetanus and antistaphylococcal gamma globulin are often used. Among the means of nonspecific immunotherapy in patients with purulent wounds, only immunomodulators are used, and only in the presence of immune disorders and always in combination with an antimicrobial drug, since they aggravate the course of the infection. Synthetic immunomodulators, such as diocephon and polyoxidonium, are the most promising. Polyoxidonium has the properties not only to restore the impaired immune response, but also to absorb toxins, and is also an antioxidant and membrane stabilizer. Usually prescribed 6 mg 2 times a week, a full course of 5-10 injections.
Symptomatic therapy includes pain relief, correction of organ and system disorders, and correction of homeostasis disorders. For pain relief, non-narcotic analgesics are usually used, however, in the early postoperative period, as well as in case of extensive injuries, narcotic drugs can be used. When the temperature rises above 39° C or fever against the background of severe diseases of the cardiovascular and respiratory systems, the prescription of antipyretic drugs is required.
¨ Prevention of infectious complications of surgical wounds
Surgical wounds are applied under conditions that minimize the risk of wound complications. In addition, before inflicting a wound, it is possible to prevent wound complications. Prevention of complications of surgical wounds includes:
· Preparation for surgery
Before a planned operation, a thorough examination of the patient is carried out, during which existing risk factors for wound complications are identified. When assessing the degree of risk, the patient’s age, nutritional status, immune status, concomitant diseases, homeostasis disorders, previous drug treatment, the condition of the tissue in the area of the proposed incision, and the type and duration of the upcoming surgical intervention are taken into account. The existing disorders are corrected and the patient is directly prepared for surgery, taking into account the requirements of asepsis.
During operations on the colon, as well as during extensive surgical interventions in extremely critically ill patients, selective intestinal decontamination is performed to prevent infectious complications. Selective intestinal decontamination reduces the risk of enterogenous infection resulting from the translocation of intestinal microorganisms. Typically a combination of an aminoglycoside or fluoroquinolone with polymyxin and amphotericin B or fluconazole is used.
With each day of hospital stay, the patient’s contamination with pathogens of hospital infections increases, so the stage of inpatient preoperative preparation should not be delayed unnecessarily.
· Careful adherence to surgical techniques
When performing surgery, careful handling of tissues, careful hemostasis, preservation of blood supply to tissues in the wound area, obliteration of the resulting “dead” space, comparison of the edges of the wound and their suturing without tension are necessary. The sutures should not be ischemic, but should ensure complete closure of the wound edges. Whenever possible, the suture material left in the wound should be absorbable and monofilament. In addition, the duration of the operation plays an important role. As it increases, the degree of wound contamination and tissue susceptibility to wound infection pathogens increases due to tissue drying, impaired blood supply, and reactive edema.
· Antibiotic prophylaxis
Antibiotic prophylaxis of infectious wound complications depends on the type of surgical procedure. In clean operations, it is indicated only in the presence of factors that adversely affect the course of the wound process, such as immunodeficiency states, diabetes mellitus, and taking immunosuppressants. For most clean and conditionally clean operations, as well as for contaminated interventions in the upper gastrointestinal tract, 1-2 generation cephalosporins, such as cefazolin or cefuroxime, can be used for antibiotic prophylaxis. For contaminated operations on the colon, biliary system and internal genital organs, the use of protected aminopenicillins or 1-2 generation cephalosporins in combination with metronidazole is indicated.
During perioperative prophylaxis, average therapeutic doses of antibiotics are used. The first dose of the drug is administered intravenously 30-60 minutes before the skin incision, usually during induction of anesthesia. If the operation lasts more than 2-3 hours, repeated administration of the antibiotic is required to maintain its therapeutic concentration in the tissues throughout the entire surgical procedure. In most cases, the duration of antibiotic administration does not exceed 24 hours, however, the presence of additional risk factors necessitates the need to extend prophylaxis to 3 days. For “dirty” interventions, a full course of antibiotic therapy is indicated, which should begin in the preoperative period.
· Antiseptic prophylaxis
Antiseptic prophylaxis involves the use of effective antiseptics at all stages of the operation, including for treating the skin, washing cavities, and subcutaneous tissue. General requirements for the antiseptics used: wide spectrum of action, high bactericidal activity, toxicological safety. To treat the skin, iodophors, chlorhexidine, and surfactants are usually used; for washing cavities, chlorhexidine, sodium hypochlorite, and dioxidine are used.
· Drainage of surgical wounds
Drainage of surgical wounds is carried out according to certain indications. It is necessary when it is impossible to obliterate the “dead space” formed after surgery, when there is a large area of the wound surface of the subcutaneous fat, when using artificial materials for plastic surgery of the aponeurosis, and in some other cases that create the preconditions for the formation of seromas. Drainage is also mandatory for radical excision of ulcers with suturing of the postoperative wound. Aspiration or flow-wash drainage is preferable, while proper care of the drainage system in the postoperative period is mandatory.
· Proper wound management in the postoperative period
Local cold is prescribed immediately after the operation, adequate pain relief, regular changes of aseptic dressings and drainage care are performed, and, if indicated, dialysis and wound evacuation, physiotherapy and other measures.
¨ Control of wound treatment
The effectiveness of wound treatment is assessed by the dynamics of general and local signs of inflammation. They focus on the subsidence of fever, leukocytosis, pain in the wound area, and normalization of the patient’s general well-being. During dressings, the condition of the sutures, the presence and extent of hyperemia and edema in the wound circumference, necrosis of the wound edges, the type of wound discharge and granulations are visually assessed. To monitor the course of the wound process in the treatment of drained wounds, instrumental research methods can be used.
An endoscopic method of examining the wound is used with simultaneous biopsy of subcutaneous fat for bacteriological examination. In this case, during dressing, an endoscope optical tube with end optics with a diameter of 3-6 mm is inserted through the drainage of the postoperative wound, the presence of wound exudate, areas of necrosis, and fibrin is assessed, then a biopsy is taken. The degree of contamination of wound tissue is determined using express methods, for example, phase-contrast microscopy. After taking a biopsy, the wound channel is filled with physiological solution to assess the correct location of the drains and the direction of the fluid flow during its jet injection.
Favorable endoscopic signs of the course of the wound process and indications for stopping drainage are: the presence of bright pink granulations, the absence of pus, necrosis, a significant amount of fibrin, tissue contamination below critical. Sluggish granulations, the presence of a large amount of exudate and fibrin in the wound, as well as high bacterial contamination require continued dialysis of the wound with antiseptic solutions.
After removal of the drainage systems, an ultrasound scan is indicated to assess the condition of the wound channel and surrounding tissues. Favorable ultrasound signs of the course of the wound process are:
· narrowing of the wound channel the next day after removal of the drainage tubes, its visualization in the form of a heterogeneous echo-negative strip by 3-5 days, absence of dilation and disappearance of the channel by 6-7 days;
· uniform echogenicity of surrounding tissues, absence of additional formations in them.
Unfavorable ultrasound signs of the course of the wound process are dilatation of the drainage channel and increased echogenicity of the surrounding tissues with the appearance of additional formations in them. These symptoms indicate the development of purulent-inflammatory wound complications even before the appearance of their clinical signs.
When treating a purulent wound, daily monitoring of the course of the wound process is necessary. With continued exudation and sluggish granulation, treatment adjustment is required. In addition to visual assessment of the condition of the wound and assessment of the severity of general clinical and laboratory symptoms, various methods are used to monitor the dynamics of the microbial landscape, the level of contamination and regenerative processes in tissues: bacteriological, cytological, modern high-precision gas-liquid chromatography, tests using enzyme systems and others.
How is such suppuration treated?
Treatment of postoperative damage begins after additional research methods have been carried out. The patient is prescribed general clinical tests. They help determine the presence of laboratory signs of wound inflammation.
Therapeutic elimination of purulent infiltrate depends on the stage of the disease. At the initial stage of suppuration formation, the surgeon cleans the wound of pathological contents, prescribes anti-inflammatory and antimicrobial drugs, and conducts detoxification therapy.
If signs of postoperative suppuration appear, which corresponds to the second stage, the wound is re-cleaned and drugs are used to heal it. The final period of the disease requires the administration of drugs to stimulate the formation of epithelium.
The main method of treating signs of postoperative suppuration is surgical treatment. The procedure is a low-traumatic type of surgical intervention. After excision of the edges of the wound, the area is carefully examined. Purulent streaks and foreign objects are detected in the diseased area. They can cause an abscess. The surgeon cuts through the leaks and removes necrotic tissue. The procedure ends with the placement of drainage at the location of the infected cavity. There are no stitches.
The pathological area with suppuration is lubricated with local antibiotics, antiseptics, and wound healing preparations. The pain is relieved with analgesics. Severe signs of intoxication postoperative syndrome are treated with antipyretic medications. When performing therapeutic measures, the situation ends with the formation of a small scar.
Need advice from an experienced doctor?
Get a doctor's consultation online. Ask your question right now.
Ask a free question
Traditional methods for eliminating signs of suppuration can be replaced with modern ones. These include vacuum therapy, laser and ozone therapy, cryodestruction, and the introduction of absorbents into the wound.
To prevent the appearance of signs of a bacteroid reaction in the form of suppuration, it is necessary to follow the rules of asepsis/antiseptics, regularly change dressings, apply stitches correctly, and buy effective drugs at the pharmacy. Ligature abscess of a postoperative scar is a reversible wound condition that has a favorable prognosis.
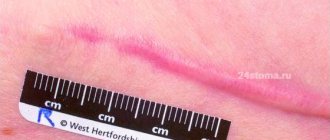
Hypertrophic and keloid scars
Scars are the result of replacement of damaged own tissues with rough connective tissue as a result of surgical interventions and various traumatic factors (mechanical, temperature, chemical, ionizing radiation, deep destructive inflammation, etc.) [1, 2, 3]. Scars, being a pronounced cosmetic defect, often lead to psycho-emotional discomfort, as well as the development of psychosocial maladjustment and a decrease in quality of life.
Scar formation goes through several stages:
- Stage 1 - inflammation and epithelization on days 7–10 after injury. The edges of the wound are connected by fragile granulation tissue; there is no scar as such yet. This period is very important for the formation of a thin and elastic scar - it is necessary to prevent suppuration and divergence of the edges of the wound.
- Stage 2 - formation of a young scar. This is 10–30 days after the injury. Collagen and elastin fibers begin to form in the granulation tissue. Increased blood supply to the injury remains - the scar is deep pink in color.
- Stage 3 - formation of a “mature” scar lasting from 1 to 3 months after injury, blood vessels completely disappear, collagen fibers line up along the lines of greatest tension. The scar becomes light and dense.
- Stage 4 - final transformation of the scar. Duration 4–12 months after injury.
Scar formation occurs mainly due to the extracellular matrix, in particular with the help of collagen. The extracellular matrix is a supromolecular complex that includes chemical compounds of various types (proteins, polysaccharides, proteoglycans, and others). It can be compared to a gel in which fibrous proteins (collagens and elastin) and viscous proteins (fibronectin, laminin) “float”, ensuring the interaction of molecules with each other and with cell surfaces [2, 9, 14, 17]. Of all proteins, collagens constitute the main component of the extracellular matrix and are the most abundant proteins in the body, accounting for about 1/3 of all its proteins. These are water-insoluble extracellular glycoproteins synthesized by fibroblasts, chondroblasts and osteoblasts. Due to the shape of the protein molecule, collagens are classified as fibrillar proteins [16]. The growth of excess extracellular matrix in the scar occurs as a result of the activity of “wound” fibroblasts. In intact (healthy) skin, fibroblasts are responsible for remodeling the components of the dermis; they destroy old collagen and lay down new one. During wounds, injuries, burns and surgical interventions, myofibroblasts appear in the wounds, which strive to “close the gap” in the tissues, intensively depositing components of the extracellular matrix: collagen, glycosaminoglycans, elastin and other proteins. It is due to the proliferation of fibroblasts and their production of excess extracellular matrix that scar growth occurs [2,19].
The following types of scars can be distinguished: normotrophic, hypertrophic and atrophic. During a normal physiological process, a normotrophic scar is formed. These are flat, light-colored scars with normal or reduced sensitivity and elasticity close to normal tissue. Such scars are optimal. In a typical wound, 6–8 weeks after injury, a balance between anabolic and catabolic processes is established. At this stage, the strength of the wound is approximately 30–40% of the strength of healthy skin. As the scar develops, its tensile strength increases as a result of the progressive bonds of collagen fibers.
When the connective tissue reacts excessively to injury against the background of unfavorable healing conditions (inflammation, scar stretching, etc.), hypertrophic scars are formed. Under conditions of hypoxia and inflammation, fibroblasts are activated by biologically active substances. Undifferentiated, pathological, functionally active cells with a high level of collagen synthesis appear. The formation of collagen prevails over its breakdown due to a decrease in the production of collagenase, a specific enzyme that destroys collagen, as a result of which powerful tissue fibrosis develops in the form of hypertrophic or keloid scars. Hypertrophic scars are often combined into a group with keloid scars due to the fact that both types are characterized by excessive formation of fibrous tissue and arise as a result of inadequate inflammation, the addition of a secondary infection, a decrease in local immunological reactions, etc. However, there are also significant differences, such as: on the basis of which differential diagnosis is carried out between these two types of scars, which will be mentioned below [3].
Atrophic scars result from a decreased response of connective tissue to injury. Not enough collagen is formed. Atrophic scars are located below the level of the surrounding skin (sink). With a small width, they practically do not differ from normotrophic ones. Often, atrophic scars appear in places of former exfoliation (acne) - the so-called “post-acne”.
Keloid (keloidum: Greek kele bulging, swelling + eidos appearance; synonym: Alibera keloid) is a dense growth of connective tissue of the skin, reminiscent of a tumor [2]. Keloid scars develop as a result of a perverted tissue reaction to injury; this is a special, most severe group of scars that differ from others in appearance and pathogenesis. As a rule, keloids form against the background of reduced levels of general and tissue immunity. When examining keloid tissue, extremely active fibroblasts are found, the degree of their activity is 4 times higher than that of cells during the normal healing process [9]. Collagen (mostly immature) is located in the form of wide loose bundles and nodes, elastin is absent. A keloid scar has an elastic consistency, an uneven, slightly wrinkled surface. Keloid is usually classified into true and false. A true (spontaneous) keloid scar occurs spontaneously without any prerequisites, most often on the skin of the chest, along the outer surface of the upper third of the shoulder, and in rare cases throughout the body. A false keloid can develop in any place where there has been previous trauma to the skin (mechanical, burn, etc.). For example, a casuistic case was observed by Chawla B., Agarwal A., Kashyap S. (2007), who diagnosed a keloid formation on the cornea of the eye after surgery. Currently, this classification is relative. Most researchers believe that spontaneous keloid is preceded by microtrauma to the skin and etiologically it is no different from a false one (in pathogenesis and morphology, these scars are absolutely identical) [2]. A keloid at the site of abrasions or scratches is a raised area. The color and intensity of the color depends on the degree of vascularization (bright pink, pale, cyanotic). A keloid scar is characterized by pulsating growth, like the ebb and flow of the tide. Scar tissue in keloids extends beyond the original wound, usually does not regress spontaneously, and tends to recur after excision.
Theoretically, keloid can appear at any age, but most often develops during and after puberty. Young individuals are more likely to be injured, and young skin creates more tension, while older skin is less elastic and more rigid. Collagen synthesis levels are also higher at younger ages. Currently, the average age of patients is 25.8 (22.3 in women and 22.6 in men) [5].
Typically, diagnosing a keloid is not difficult. Gulamhuseinwala N., Mackey S., Meagher P. (2008), who conducted a retrospective study of 568 patients with keloids, found that the clinical diagnosis coincided with the histological diagnosis in 94% of cases. However, when scars begin to appear, the question of differential diagnosis between hypertrophic scar and keloid arises. As mentioned above, there are significant differences between these types of scars. The growth of a hypertrophic scar begins immediately after healing and is characterized by the formation of “plus tissue”, an area equal to the wound surface, while the boundaries of the keloid always extend beyond the damaged area. With a hypertrophic scar, subjective sensations are absent or insignificant. Keloid formations cause various subjective sensations (itching, pain, feeling of skin tightness, parasthesia, etc.). The change in color of a hypertrophic scar from pink to whitish occurs at the same time as in normotrophic scars, and over time they noticeably flatten. The keloid remains deep in color and does not regress spontaneously. Typically, one patient has several keloid formations. Keloid scars are most common in people aged 10–30 years, while hypertrophic scars can occur at any age. The morphological picture also has significant differences. It is known that collagen synthesis in keloids is approximately 8 times higher than in hypertrophic scars, which explains the lower quantitative content of collagen fibers in hypertrophic scars, and therefore the mass of the scar. In hypertrophic scars there are fewer fibroplastic cells than in keloids, and there are no giant, immature forms of the “growth zone” [1, 7, 8, 9, 12, 21].
Researchers have noted an unequal frequency of localization of keloid formation in individuals of different races. For example, in white-skinned people, keloids often form on the face (cheek and ear), upper extremities and sternal area; and in Asians the sternal region is almost not affected; in blacks, along with the auricle and neck, the lower extremities are more often affected [5]. This suggested the leading role of heredity in keloid formation. However, to date, no specific gene or set of genes has been identified as being responsible for keloid development [5, 8]. In addition, no significant differences were found between keloid DNA fibroblasts and normal skin DNA fibroblasts. This suggests that what is important in the development of keloid formation is not the increase in the number of fibroblasts producing normal amounts of collagen, but rather that each fibroblast produces more collagen than is necessary for wound repair [13].
Treatment of keloid and hypertrophic scars
The first rule in the treatment of keloids and hypertrophic scars is their prevention. First, unnecessary cosmetic surgery should be avoided in patients prone to keloid formation and severe scarring. A possible exception would be a disfiguring keloid on the pinna. If surgery cannot be avoided, closure of all surgical wounds should occur with minimal tension along the skin folds whenever possible. It is advisable that the incisions do not pass through the surface of the joints and in the middle part of the chest.
Treatment methods for keloid and hypertrophic scars can be divided into several groups:
- medications (corticosteroids, immunomodulators, drugs affecting collagen formation);
- physical and physiotherapeutic (use of occlusive dressings and compression therapy, excision, cryosurgery, laser therapy, electrophoresis, etc.);
- radiation therapy;
- cosmetic procedures aimed at external correction of the defect.
Medicines
Corticosteroids. Intraruminal steroids remain the mainstay of treatment. Corticosteroids reduce scar formation by reducing the synthesis of collagen, glycosaminoglycans, inflammatory mediators, and fibroblast proliferation during wound healing. The most commonly used corticosteroid is triamcinolone acetate at a concentration of 10–40 mg/mL, administered into the injured area by needle injection at 4–6 week intervals. The effectiveness of such an introduction as a monomodel and as an addition to the scar excision procedure is very high. Topical corticosteroids are also widely used, which are applied daily directly to the formation. Complications of corticosteroid treatment include atrophy, telangiectasias, and pigmentation disorders.
Immunomodulators. A new method in the treatment of keloid and hypertrophic scars is interferon therapy. Interferon injected into the suture line after excision of a keloid scar can prophylactically prevent relapses. It is recommended to administer 0.5–1.0 million IU every other day for 2–3 weeks, then 0.1–0.5 million IU 1–2 times a week for three months.
Drugs that reduce hyperproliferation of connective tissue cells. The classic treatment for scars is hyaluronidase. Hyaluronidase breaks down the main component of the interstitial substance of connective tissue - hyaluronic acid, which is a cementing substance of connective tissue, and thus increases tissue and vascular permeability, facilitates the movement of fluids in the interstitial spaces; reduces tissue swelling, softens and flattens scars, and prevents their formation. Preparations containing hyaluronidase (Lidase and Ronidase) are obtained from the testes of cattle. Lidase solution (1 ml) is administered in these cases near the site of the lesion under the skin or under scar tissue. Injections are made daily or every other day; the course of treatment consists of 6–10–15 or more injections. If necessary, repeat courses are carried out at intervals of 1.5–2 months.
A new enzyme preparation for the treatment of diseases accompanied by the growth of connective tissue is Longidaza. "Longidase" is a chemical compound of hyoluronidase with polyoxidonium. The combination of the enzymatic activity of hyaluronidase with the immunomodulatory, antioxidant and moderate anti-inflammatory properties of polyoxidonium provides a wide range of pharmacological properties. It is most effective to use the drug "Longidaza" by ultraphonophoresis or phonophoresis. For ultraphonophoresis, Longidase 3000 IU is diluted in 2–5 ml of gel for ultrasound therapy. The impact is carried out with a small ultrasonic emitter (1 cm2), with an ultrasound frequency of 1 MHz, intensity 0.2–0.4 W/cm2, in continuous mode, exposure time 5–7 minutes, course of 10–12 procedures daily or every other day . Using the phonophoresis method (1500 Hz), 3000 IU of Longidase is administered daily (total exposure time 5 minutes, course - 10 procedures). It is also possible to administer the drug inside the scar:
- small keloid and hypertrophic scars: Longidaza 3000 IU once every 7 days for a total course of 10 injections into the scar;
- keloid and hypertrophic large area of the lesion: Longidase 3000 IU 1 time in 7 days inside the scar in a course of 8-10 injections, at the same time intramuscular injection of Longidase 3000 IU No. 10.
A well-known drug that inhibits the proliferation of connective tissue cells and at the same time has an anti-inflammatory effect is Contractubex. For many years, Contractubex has been used in surgery and cosmetology in the treatment of postoperative and post-burn scars, including rough scars that impede movement and keloids, as well as stretch marks (striae) after childbirth or after sudden weight loss.
The enzyme preparation of 9 collagenolytic proteases “Fermenkol” is a fundamentally new proteolytic drug. The anti-scar effect of Fermenkol is based on the reduction of excess extracellular matrix in scar tissue. The effect when using anti-scarring agents is observed approximately 3 weeks after the start of use of the product and the optimal result is usually achieved after 2-3 courses of electrophoresis or phonophoresis, 10-15 sessions or applications for 30-60 days.
Physical and physiotherapeutic methods
Silicone sheets and silicone occlusive dressings have been used with varying degrees of success in the treatment of hypertrophic scars and keloids. When using silicone dressings, the effect is more a result of a combination of pressure and surface hydration than the effect of the silicone itself. Studies show that the effectiveness of such dressings when used 24 hours a day for 12 months was observed in only ≈1/3 of patients [20]. The compression/pressure method achieved with silicone sheets causes thinning of the skin. In one study, applying two plates to the earlobes after keloid removal prevented its recurrence for 8 months to 4 years. A significant discomfort of the method is the need to wear bandages/plates throughout the day, as well as the impossibility of attaching them in some anatomical areas (folds, neck, etc.). Other devices (special compression garments and zinc oxide patches) are also used 12 to 24 hours a day for 8 to 12 months, which is a clear disadvantage of the method. According to various authors, their effectiveness does not exceed 40% [4, 5].
Cryosurgery. Cryosurgical agents, such as liquid nitrogen, attack the microvasculature and cause cell death through the formation of intracellular crystals. Typically, 1–3 freeze-thaw cycles of 10–30 seconds are sufficient to achieve the desired effect. Particular attention should be paid to the short duration of liquid nitrogen applications to avoid the appearance of reversible hypopigmentation. Please note that cryotherapy can be painful and cause depigmentation. A more promising method is the combined effect of cryotherapy and corticosteroids [15]. A simple and effective method of facilitating intraruminal steroid injection is to apply very light cryotherapy prior to injection, a step that will cause tissue swelling and subsequent cellular and collagen destruction. Liquid nitrogen is applied for 5-15 seconds, without allowing the skin to freeze. This technique allows for better distribution of the drug in the keloid tissue and minimizes its penetration into surrounding tissues.
Laser therapy. Argon laser (488 nm) is similar to carbon dioxide laser and can promote collagen contraction through local heating. A pulsed dye laser (585 nm) causes a process of photothermolysis, leading to microvascular thrombosis. In the early 1980s, authors noted that with the use of pulsed dye laser, scars became less erythematous, softer, and less hypertrophic. Laser treatment of keloids and hypertrophic scars using a carbon dioxide laser (10,600 nm) can ablate (can cut and cauterize) the lesion, creating a dry surgical environment with minimal tissue trauma. When carbon dioxide laser is used as a single model, the recurrence rate of keloid is quite high, so it is recommended to combine laser treatment with postoperative steroid administration, which significantly reduces the recurrence rate.
Radiation therapy. The use of radiation therapy to treat keloids remains controversial. One retrospective study found that the use of superficial radiation therapy after scar resection caused recurrence in 53% of patients [18]. Preoperative treatment of scars with a solution of hyaluronidase, followed by surgical removal and then external irradiation, is considered more promising to reduce the likelihood of relapse [6].
X-ray therapy (Bucchi rays) is based on the action of ionizing radiation on connective tissue, causing swelling and destruction of collagen fibers and fibroblasts. X-ray therapy is prescribed up to 6 irradiation sessions with an interval of 6–8 weeks at a single dose of up to 15,000 R. Only the superficial layers of the skin (in particular, the scar) are exposed to ionizing radiation, and the X-ray load on the underlying tissue is insignificant. Contraindications to Bucca therapy are kidney disease, circulatory decompensation, the presence of dermatitis and residual wounds. The idea of using x-ray therapy is very rational, because in the case of destruction of a certain number of fibroblasts, a balance is achieved between the synthesis and degradation of complex collagen, up to a change in its very structure. This idea, in particular, has been implemented using modern laser technology. The total radiation dose to prevent recurrence of a keloid is 15 to 20 Gy. They also recommend one-time irradiation of the wound on the day of suture removal in the same doses - 15–20 Gy. Sometimes this procedure is repeated up to 6 times at intervals of 1.5–2 months.
Excision. It is advisable to avoid surgery, especially if a keloid is present. Excision relapses in 45–100% of cases and should only rarely be used as a standalone model of therapy. After excision, long-term anti-relapse therapy is necessary. Whenever possible, wound pressure bandages and linens are used in the early postoperative period. Reducing relapses is achieved by combining excision with other methods, such as radiation or radiotherapy, interferon or corticosteroids [10].
After surgical interventions or skin injuries, it is necessary to take preventive measures to reduce the risk of keloid formation or hypertrophic sutures. The period of active therapy should begin at the 2nd stage of scar formation, i.e. 10–30 days after injury. According to the authors, there is a proportional dependence on the effectiveness of therapeutic treatment on the age of the scars: scars older than 6 months are less responsive to therapy than younger ones. Most likely, the decrease in the effectiveness of therapeutic measures is associated with a decrease in the number of vessels and compaction of scar tissue, which worsens its trophism [3]. Preventive methods may include: occlusive dressings, compression therapy, external applications or intradermal administration of glucocorticosteroids, Contractubex, Longidase, immunotherapy.
With a formed scar with a duration of up to 12 months, it is possible to carry out therapy with all methods, and with a long-term formation (more than 12 months), only aggressive methods are effective: injection of corticosteroids into the affected area, excision, radiation therapy, Bucca therapy, laser therapy.
Cosmetic procedures aimed at external correction of the defect. The most popular cosmetic procedures (peelings, mesotherapy, dermabrasion) do not serve any therapeutic purpose when affecting scars. The use of these methods contributes to the aesthetic correction of small scars. It is important to know that hypertrophic scars can be resurfaced only after they have reached a calm state. To obtain satisfactory results in the treatment of scars with a duration of more than six months, cosmetic procedures must be combined with 1–2 injections of corticosteroids (Diprospan) [2]. Sandblasting dermabrasion (10–15 sessions with an interval of 10–14 days) with a standard course of treatment provides satisfactory results, but when conducting two courses of 10–15 procedures, the effectiveness of treatment increases [2]. Surgical dermabrasion of hypertrophic scars requires re-operation after six months. Complete epithelization of hypertrophic scars after deep surgical dermabrasion occurs much later than epithelization of normotrophic and hypotrophic scars and lasts for 3–4 weeks, even when using wound healing ointments [2]. O. S. Ozerskaya (2002) performed a transplantation of autologous keratinocytes to prevent recurrence of scars after dermabrasion. Unhindered removal of the bandages is possible on days 7–8; complete epithalization of the scars occurred within up to 14 days. Thus, a significant acceleration of epithalization during dermabrasion of scars with keratinocyte transplantation is obvious. To avoid even greater hypertrophy and hyper- or depigmentation, hypertrophic scars cannot be corrected using aggressive methods, and medium peels can be done only after a course of superficial peels. As for keloid scars, it is better not to expose them to irritating methods, since there is a high probability of provoking their further growth.
Peeling with fruit acids. During peeling, dead skin cells are exfoliated and the regeneration processes of its deep layer are stimulated. Fruit acids gently penetrate the skin without damaging it and stimulate the production of collagen and elastin. Thanks to this, the elasticity of the skin increases, the relief is evened out and hyperpigmentation is reduced, which externally corrects the cosmetic defect of small scars.
Chemical peels. For small, mildly expressed hypertrophic scars, chemical peels are best performed in two stages. At the same time, in daily home care on the 21st day, in order to smooth out scar hypertrophy, a silicone dermatological cream is included, which creates an occlusive film, protecting the skin from moisture loss, relieves inflammation and erythema, and stimulates repair. In parallel with peelings, you can conduct mesotherapy sessions with regenerants and local microcirculation enhancers. Treatment of mildly expressed hypertrophied scars after a course of chemical peels can be continued with topical medications. For severe hypertrophic scars, peelings are carried out six to eight months after excision of the pathological scar or a course of physiotherapy (galvano-, phonophoresis) with hyaluronidase and heparin. The course of chemical peelings is also carried out in two stages: first, several sessions of multi-fruit peels (glycolic, salicylic, lactic, citric acids) once a week, then a complex retinol yellow peeling. Peeling is performed according to an individual scheme in accordance with the parameters of the scar and skin reaction.
In conclusion, it should be noted that the therapy of keloid and hypertrophic scars, with all the variety of treatment methods, requires a strictly individual approach, taking into account the main parameters of the scar: the size and duration of its existence.
For questions regarding literature, please contact the editor.
Yu. A. Gallyamova*, Doctor of Medical Sciences Z. Z. Kardashova** * RMAPO, ** Russian State Scientific Center, Moscow
Key words: hypertrophic scars, keloid scars, keloid disease, keloid, keloid formation, keloid treatment, scar treatment, scar prevention.
Is suppuration dangerous and its course?
Postoperative scar abscess requires early diagnosis of signs and timely initiation of conservative therapy. In a short period of time, with adequate treatment, pus is removed and the proliferation of pathogenic microorganisms is suppressed. Tissue regeneration in a cleaned wound occurs quickly.
The danger of an untreated abscess is the manifestation of signs of postoperative complications. Suppuration can develop into phlegmon or fistula. Both pathologies require surgical intervention. With advanced inflammation of the wound, there is a high probability of developing sepsis. The disease is characterized by the patient's severe condition and severe intoxication. A septic reaction develops when bacteria and their metabolic products enter the bloodstream. The pathological situation can be fatal.
To prevent the appearance of signs of complications, it is necessary to treat suppuration in the early stages of development.
Publications in the media
Wound is an injury to any part of the body (especially caused by physical impact), manifested by a violation of the integrity of the skin and/or mucous membrane.
Classification • By etiology •• Puncture wound - a wound inflicted by a sharp object with small transverse dimensions (characterized by a narrow and long wound channel) •• Gunshot wound - damage to tissues and organs with a violation of the integrity of their cover (skin, mucous or serous membrane), caused by gunshot wounding projectile and characterized by a zone of primary necrosis and changes that cause the formation of foci of secondary necrosis in the surrounding tissues •• Operational (surgical) wound - a wound inflicted during a surgical operation •• Crushed wound - a wound with an extensive zone of primary traumatic necrosis, during the application of which occurred crushing and rupture of tissue •• Lacerated wound - a wound that occurs under the influence of overextension of tissue •• Cut wound - a wound caused by the sliding movement of a thin sharp object •• Chopped wound - a wound caused by a blow with a heavy sharp object; characterized by a large depth, insignificant zone of primary traumatic necrosis •• Scalped wound - a wound with complete or partial separation of a large flap of skin (on the scalp - all soft tissues) •• Bite wound - a wound inflicted by the teeth of an animal or person; characterized by infection, uneven crushed edges •• Contused wound - a wound from a blow with a blunt object with simultaneous bruising of surrounding tissues • According to pathomorphology •• Aseptic wound - a wound that practically does not contain microorganisms •• Bacterially contaminated wound - a wound containing microorganisms that do not cause (or have not yet caused) pathological changes •• Purulent wound - a wound characterized by purulent inflammation of the walls and bottom of its cavity •• Granulating wound - a wound whose cavity is filled with granulation tissue (heals by secondary intention) •• Infected wound - a wound containing pathogenic microorganisms that caused wound infection •• A poisoned wound is a wound that contains poison. Clinical picture • Pain • Gaping of the skin defect • Bleeding. Emergency care : bleeding control, pain relief, aseptic dressing, immobilization.
Treatment • Primary surgical treatment - excision of necrotic tissue and wound edges, suturing •• Fresh wounds - application of a primary blind suture •• In case of late treatment or in the presence of infection, a secondary suture is applied after cleansing the wound • Emergency prevention of tetanus . One of the following drugs is used: adsorbed tetanus toxoid (AS-toxoid), antitetanus human immunoglobulin, antitetanus serum. Complications • Traumatic shock • Blood loss • Soft tissue infection.
ICD-10. T14.0 Superficial trauma to unspecified area
The International Statistical Classification of Diseases and Problems, 10th revision (ICD-10), currently in use, is a normative document that ensures the formation of unified statistical control over the activities of medical institutions, assessment of the epidemiological situation in the country, and allows for the unity and comparability of incoming medical data [1] . ICD-10 is one of the important methodological tools for processing statistical data, which is extremely necessary for automating basic planning, regulatory and management work [2]. Unfortunately, this classification contains errors, both in the structural design of clinical sections and in the coding of a number of diseases. Some of the diagnoses that exist in it sound somewhat absurd; there is a confusion of placement of individual polyetiological diseases and syndromes in sections, which are often located in chapters that have a very indirect relationship to this nosology. In addition, many nosological forms, syndromes and symptoms that are currently common are not included in the accepted classification, which undoubtedly significantly limits the practical application of ICD-10.
The variety of different forms of diseases complicates the structure of the classification, so the latest revision has become much larger in volume and was accompanied by extensive guidelines, unfortunately, often without taking into account clinical practice. As a result, some of the clinical diagnoses ended up within the limits of unrefined conditions or conditions that were not sufficiently differentiated, and not in the headings or subheadings of the corresponding sections of the classification.
A number of difficulties arise with regard to the topographic classifications and terms used in ICD-10, many of which are imprecise, contradictory and do not correspond to clinical practice. Unfortunately, it should be noted that ICD-10 cannot currently be recommended as a model for terminology and recording of clinical diagnoses in medical records and other medical records.
To solve the problem of uniform standardization of medical diagnoses and statistical records, back in the late 70s of the last century, the idea arose of creating a “family” of classifications of diseases and health problems based on the ICD, adapted to a specific specialty, taking into account modern achievements of medical science.
Classification options for dentists, oncologists, dermatologists, rheumatologists and orthopedists, pediatricians, psychiatrists, and general practitioners have been published and recommended by WHO. Unfortunately, in the field of andrology there is still no unified classification [2].
Currently, the Research Institute of Urology is developing printed and electronic versions of a similar classification for urology and andrology, which is based on three-digit and four-digit ICD-10 codes. The coding system for urological and andrological diseases is presented in the form of a tree, where each subnode refines the information of the previous node. When forming a subnode, a number from zero to nine is added to the main code through a dot. The proposed system also provides for taking into account the localization of the pathological process, its stage and/or phase [3-5].
The creation of a printed and electronic version of a new unified clinical and statistical classification of andrological diseases will allow:
- facilitate data collection and improve the analysis of statistical material on andrology by increasing the reliability of statistical information and reducing the number of errors due to inconsistency of clinical diagnoses with ICD-10 diagnoses;
- provide a detailed assessment of the complexity of clinical cases and final treatment results when providing specialized (including high-tech) medical care;
- standardize the formulation of andrological diagnoses in medical documents and computer information systems of health authorities of the Russian Federation;
- improve the economic and statistical analysis of the provision of medical care in the field of andrology in the interests of healthcare of the Russian Federation, individual medical institutions and compulsory health insurance (CHI);
- optimize costs for diagnosis and treatment of andrological diseases.
It should be taken into account that andrological diseases, as a rule, are multi-etiological and many of them do not fit only into the framework of the block “Diseases of the male genital organs”. The modern concept of andrology is, of course, based on interdisciplinary interaction and is at the intersection of related medical disciplines (urology, endocrinology, psychiatry, genetics, neurology, therapy, etc.). Therefore, andrological diseases are heterogeneous in nature and are widely represented in different sections of ICD-10 (Table 1).
Table 1. General classification of andrological diseases according to ICD-10 and proposals for its correction
| ICD-10 code | Nosology | Correction and comments |
| 1 | 2 | 3 |
| A54.0 | Gonococcal infection of the lower genitourinary tract without abscessation of the periurethral or accessory glands | |
| A54.1 | Gonococcal infection of the lower genitourinary tract with abscess formation of the periurethral and accessory glands | |
| A56 | Other chlamydial sexually transmitted diseases | |
| A56.0 | Chlamydial infections of the lower genitourinary tract | |
| A56.1 | Chlamydial infections of the pelvic organs and other genitourinary organs | |
| A56.2 | Chlamydial infection of the genitourinary tract, unspecified | |
| A56.8 | Chlamydial infections, sexually transmitted infections, other localization | |
| A59 | Trichomoniasis | |
| A59.0 | Urogenital trichomoniasis | |
| A60 | Anogenital herpetic viral infection (herpes simplex) | |
| A60.0 | Herpetic infections of the genitals and genitourinary tract | |
| D17.6 | Benign neoplasm of adipose tissue of the spermatic cord | |
| E28.0 | Excess estrogen | Controversial diagnosis. It has no clinical use in men. |
| E28.1 | Excess androgens | Controversial diagnosis. Not all specialists accept the upper limits of normal. |
| E29 | Testicular dysfunction | Controversial diagnosis. Has no clinical use. |
| E29.0 | Testicular hyperfunction | Controversial diagnosis. Has no clinical use. |
| E29.1 | Testicular hypofunction | Controversial diagnosis. Has no clinical use. |
| E29.8 | Other types of testicular dysfunction | Controversial diagnosis. Has no clinical use. |
| E29.9 | Testicular dysfunction, unspecified | Controversial diagnosis. Has no clinical use. |
| E89.5 | Testicular hypofunction after medical procedures | Controversial diagnosis. Has no clinical use. |
| F52 | Sexual dysfunction not due to organic disorders or diseases | We propose to reduce this diagnosis due to its uncertainty, and refer all cases of erectile dysfunction to paragraph N48.4. |
| F52.0 | Lack or loss of sexual desire | |
| F52.1 | Aversion to sexual intercourse and lack of sexual pleasure | |
| F52.2 | Lack of genital response | Controversial diagnosis. It has no clinical use in men. |
| F52.3 | Orgasmic dysfunction | |
| F52.4 | Premature ejaculation | Correction: F52.4: Ejaculation disorders |
| Correction: F52.4.0 Premature ejaculation. | ||
| Correction: F52.4.1 Delayed ejaculation. | ||
| Correction: F52.4.2 Retrograde ejaculation. | ||
| Correction: F52.4.3 Anejaculation. | ||
| F64.0 | Transsexualism | |
| F64.2 | Gender identity disorder in childhood | |
| F64.8 | Other gender identity disorder | |
| F64.9 | Gender identity disorder, unspecified | |
| F65 | Sexual preference disorders | |
| F65.6 | Multiple disorders of sexual preference | |
| F65.8 | Other disorders of sexual preference | |
| F65.9 | Disorder of sexual preference, unspecified | |
| F66 | Psychological and behavioral disorders associated with sexual development and orientation | |
| F66.0 | Sexual maturation disorder | |
| F66.1 | Egodystonic sexual orientation | |
| F66.2 | Sexual relationship disorder | Controversial diagnosis. Correction: sexual disharmony. |
| F66.8 | Other psychosexual developmental disorders | |
| F66.9 | Psychosexual development disorder, unspecified | |
| F68.0 | Exaggeration of somatic symptoms for psychological reasons | |
| F83 | Mixed specific psychological development disorders | |
| F84 | General psychological disorders | |
| I86.1 | Varicose veins of the scrotum (varicocele) | |
| Correction: I86.1.0 Varicocele on the left. | ||
| Correction: I86.1.1 Varicocele on the right. | ||
| Correction: I86.1.2 Varicocele hemodynamic type 1 (renotesticular type of reflux). | ||
| Correction: I86.1.3 Varicocele hemodynamic type 2 (ileotesticular type of reflux). | ||
| Correction: I86.1.4 Varicocele hemodynamic type 3 (mixed type of reflux). | ||
| Correction: I86.1.5 Bilateral varicocele. | ||
| N34.0 | Urethral abscess | |
| N34.1 | Nonspecific urethritis | |
| N34.2 | Other urethritis | |
| N35 | Urethral stricture | |
| N35.0 | Post-traumatic urethral stricture | |
| N35.1 | Post-infectious urethral stricture, not elsewhere classified | |
| N35.8 | Other urethral stricture | |
| N35.9 | Urethral stricture, unspecified | |
| N36 | Other urethral diseases | |
| N36.0 | Urethral fistula | |
| N36.1 | Urethral diverticulum | |
| Correction: N36.2 Stenosis of the external urethral meatus. | ||
| Correction: N36.3 Obliteration of the urethra. | ||
| N36.8 | Other specified diseases of the urethra | |
| N36.9 | Urethral disease, unspecified | |
| N37* | Lesions of the urethra in diseases classified elsewhere | |
| N37.0* | Urethritis in diseases classified elsewhere | |
| N37.8* | Other lesions of the urethra in diseases classified elsewhere | |
| N39 | Other diseases of the urinary system | |
| N39.0 | Urinary tract infection without established localization | |
| N39.3 | Involuntary urination | |
| N39.4 | Other specified types of urinary incontinence | |
| N39.8 | Other specified diseases of the urinary system | |
| N39.9 | Urinary system disorder, unspecified | |
| N40 | Benign prostatic hyperplasia | |
| Correction: N40.0 With impaired quality of urination. | ||
| Correction: N40.1 Without affecting the quality of urination. | ||
| N41.0 | Acute prostatitis | |
| N41.1 | Chronic prostatitis | |
| Correction: N41.1.0 Chronic bacterial prostatitis. | ||
| Correction: N41.1.1 Chronic abacterial prostatitis. | ||
| Correction: N41.1.1.1 Abacterial CP with the presence of inflammation. | ||
| Correction: N41.1.1.2 Abacterial CP without signs of inflammation. | ||
| Correction: N41.1.2 Asymptomatic chronic prostatitis. | ||
| N41.2 | Prostate abscess | |
| N41.3 | Prostatocystitis | Controversial diagnosis. Has no clinical use. We propose to reduce this diagnosis. Correction: N41.3 Colliculitis. |
| Correction: N41.4 Sclerosis of the prostate gland. | ||
| N41.8 | Other inflammatory diseases of the prostate gland and seminal vesicles | |
| N41.9 | Inflammatory disease of the prostate, unspecified | |
| N42 | Other prostate diseases | |
| N42.0 | Prostate stones | Controversial diagnosis. Has no clinical use. We propose to reduce this diagnosis. Correction: Simple prostate cyst. |
| N42.1 | Congestion and hemorrhage in the prostate gland | Controversial diagnosis. Has no clinical use. We propose to reduce this diagnosis. |
| N42.2 | Prostate atrophy | |
| N42.8 | Other specified prostate diseases | |
| N42.9 | Prostate disease, unspecified | |
| N43 | Hydrocele and spermatocele | We propose in paragraph N43 to consider only hydrocele. |
| N43.0 | Hydrocele encyscum | Controversial diagnosis. Has no clinical use. We propose to reduce this diagnosis. Correction: Hydrocele on the left. |
| N43.1 | Infected hydrocele | Correction: Hydrocele on the right. |
| N43.2 | Other forms of hydrocele | Correction: Infected hydrocele. |
| N43.3 | Hydrocele, unspecified | Correction: Uninfected hydrocele. |
| N43.4 | Spermatocele | Correction: Hydrocele, unspecified. |
| Correction: N43.5 Spermatocele. | ||
| N44 | Testicular torsion | Correction: N44.0 Testicular torsion. |
| Correction: N44.1 Torsion of Morgagni's hydatid. | ||
| N45 | Orchitis and epididymitis | |
| N45.0 | Orchitis, epididymitis and epididymo-orchitis with abscess | Correction: Unilateral orchitis, epididymitis and epididymo-orchitis with abscess. |
| N45.9 | Orchitis, epididymitis and epididymo-orchitis without mention of abscess | Correction: N45.1 Unilateral orchitis, epididymitis and epididymo-orchitis without abscess. |
| Correction: N45.2 Bilateral orchitis, epididymitis and epididymo-orchitis with abscess. | ||
| Correction: N45.3 Bilateral orchitis, epididymitis and epididymo-orchitis without abscess. | ||
| N46 | Male infertility | Due to the polyetiological nature of the factors leading to male infertility, we propose to differentiate these forms depending on the causative factors. |
| Correction: N46.0 Male infertility caused by dysfunction at the level of the hypothalamus/pituitary gland. | ||
| Correction: N46.1 Male infertility caused by dysfunction at the testicular level. | ||
| Correction: N46.2 Male infertility caused by dysfunction of the efferent seminal tract and accessory sex glands. | ||
| Correction: N46.3 Male infertility caused by an ejaculation defect. | ||
| Correction: N46.4 Male infertility caused by an immunological factor. | ||
| Correction: N46.5 Male infertility caused by dysfunction of other androgen target organs. | ||
| Correction: N46.6 Male infertility, idiopathic form. | ||
| ALTERNATIVE CLASSIFICATION OF MALE INFERTILITY | ||
| Correction: N46.0 Male infertility is caused by a damaging external factor. | ||
| Correction: N46.1 Male infertility is caused by chromosomal pathology. | ||
| Correction: N46.2 Male infertility is caused by an anomaly in the structure or development of the male genital organs. | ||
| Correction: N46.3 Male infertility is caused by an endocrine factor. | ||
| Correction: N46.4 Male infertility is caused by infectious and inflammatory diseases of the male genital organs. | ||
| Correction: N46.5 Male infertility is caused by an immunological factor. | ||
| Correction: N46.6 Male infertility is caused by an iatrogenic factor. | ||
| Correction: N46.7 Male infertility is caused by an isolated pathology of seminal plasma. | ||
| Correction: N46.8 Idiopathic male infertility. | ||
| N47 | Excessive foreskin, phimosis and paraphimosis | Correction: N47.0 Excessive foreskin. |
| Correction: N47.1 Phimosis. | ||
| Correction: N47.2 Paraphimosis. | ||
| Correction: N47.3 Functional paraphimosis. | ||
| N48 | Other diseases of the penis | |
| N48.0 | Leukoplakia of the penis | |
| N48.1 | Balanoposthitis | |
| Correction: N48.1.0 Acute balanoposthitis. | ||
| Correction: N48.1.1 Chronic balanoposthitis. | ||
| N48.2 | Other inflammatory diseases of the penis | |
| Correction: N48.2.0 Cavernit. | ||
| Correction: N48.2.1 Serous cavernitis. | ||
| Correction: N48.2.2 Purulent cavernitis. | ||
| Correction: N48.2.3 Cavernous fibrosis. | ||
| N48.3 | Priapism | |
| Correction: N48.3.0 Arterial priapism. | ||
| Correction: N48.3.1 Ischemic priapism. | ||
| Correction: N48.3.2 Nocturnal intermittent priapism. | ||
| N48.4 | Impotence of organic origin | We suggest making a correction: Psychosomatic syndrome of erectile dysfunction. |
| Correction: N48.4.0 Erectile dysfunction with a predominance of the organic component. | ||
| Correction: N48.4.1 Erectile dysfunction with a predominance of the psychogenic component. | ||
| N48.5 | Penile ulcer | |
| N48.6 | Balanitis | |
| Correction: N48.6.0 Acute balanitis. | ||
| Correction: N48.6.1 Chronic balanitis. | ||
| Correction: N48.6.2 Xerotic balanitis obliterans. | ||
| N48.8 | Other specified diseases of the penis | |
| Correction: N48.8.0 Small penis. | ||
| Correction: N48.8.1 Ectopia of the penis. | ||
| Correction: N48.8.2 Double penis. | ||
| Correction: N48.8.3 Webbed penis. | ||
| Correction: N48.8.4 Retractile penis. | ||
| Correction: N48.8.5 Hidden penis. | ||
| Correction: N48.8.6 Peyronie's disease. | ||
| Correction: N48.8.6.0 Peyronie's disease in the active stage. | ||
| Correction: N48.8.6.1 Peyronie's disease in the functional stage. | ||
| N48.9 | Disease of the penis, unspecified | |
| N49 | Inflammatory diseases of the male genital organs, not elsewhere classified | |
| N49.0 | Inflammatory diseases of the seminal vesicle | Correction: Vesiculitis. |
| Correction: N49.0.0 Acute vesiculitis. | ||
| Correction: N49.0.1 Chronic vesiculitis. | ||
| N49.1 | Inflammatory diseases of the spermatic cord, tunica vaginalis and vas deferens | |
| N49.2 | Inflammatory diseases of the scrotum | |
| Correction: N49.3 Fournier's gangrene. | ||
| N49.8 | Inflammatory diseases of other specified male genital organs | |
| N49.9 | Inflammatory diseases of the unspecified male genital organ | |
| N50 | Other diseases of the male genital organs | |
| N50.0 | Testicular atrophy | |
| N50.1 | Vascular disorders of the male genital organs | Controversial diagnosis. Has no clinical significance. |
| Correction: N50.2 Contact dermatitis of the genitals. | ||
| N50.8 | Other specified diseases of the male genital organs | |
| N50.9 | Male genital disease, unspecified | |
| N51* | Lesions of the male genital organs in diseases classified elsewhere | |
| N51.0* | Lesions of the prostate gland in diseases classified elsewhere | |
| N51.1* | Lesions of the testicle and its appendages in diseases classified elsewhere | |
| N51.2* | Balanitis in diseases classified elsewhere | |
| N51.8* | Other lesions of the male genital organs in diseases classified elsewhere | |
| N51.8* | Other lesions of the male genital organs in diseases classified elsewhere | |
| N94.1 | Dyspareunia | |
| N99 | Disorders of the genitourinary system after medical procedures not classified elsewhere | |
| N99.1 | Postoperative urethral stricture | |
| N99.8 | Other disorders of the genitourinary system after medical procedures | |
| N99.9 | Disorders of the genitourinary system after medical procedures, unspecified | |
| Q53 | Undescended testicle | |
| Q53.0 | Ectopic testicle | |
| Q53.1 | Unilateral undescended testicle | |
| Q53.2 | Bilateral undescended testicle | |
| Correction: Q53.3 Pseudocryptorchidism. | ||
| Q53.9 | Undescended testicle, unspecified | |
| Q54 | Hypospadias | |
| Q54.0 | Hypospadias of the glans penis | |
| Q54.1 | Penile hypospadias | |
| Q54.2 | Hypospadias penis-scrotum | |
| Q54.3 | Perineal hypospadias | |
| Q54.4 | Congenital curvature of the penis | |
| Q54.8 | Other hypospadias | Correction: Hypospadias without hypospadias. |
| Q54.9 | Hypospadias, unspecified | |
| Q55 | Other congenital anomalies [malformations] of the male genital organs | |
| Q55.0 | Absence and aplasia of the testicle | |
| Q55.1 | Testicular and scrotal hypoplasia | |
| Q55.2 | Other congenital anomalies of the testicle and scrotum | |
| Correction: Q55.2.0 Monorchidism. | ||
| Correction: Q55.2.0.0 Monorchidism on the right. | ||
| Correction: Q55.2.0.1 Monorchidism on the left. | ||
| Correction: Q55.2.1 Polyorchidism. | ||
| Correction: Q55.2.2 Synorchidism. | ||
| Q55.3 | Atresia of the vas deferens | |
| Q55.4 | Other congenital anomalies of the vas deferens, epididymis, spermatic cord and prostate | |
| Q55.5 | Congenital absence and aplasia of the penis | |
| Q55.6 | Other congenital anomalies of the penis | |
| Q55.8 | Other specified congenital anomalies of the male genital organs | |
| Q55.9 | Congenital anomaly of the male genital organs, unspecified | |
| Q56 | Sex ambiguity and pseudohermaphroditism | |
| Q56.0 | Hermaphroditism, not elsewhere classified | |
| Q56.1 | Male pseudohermaphroditism, not elsewhere classified | |
| Q56.3 | Pseudohermaphroditism, unspecified | |
| Q56.4 | Gender ambiguity, unspecified | |
| Q64 | Other congenital anomalies [malformations] of the urinary system | |
| Q64.0 | Epispadias | |
| Correction: Q64.0.0 Epispadias of the head. | ||
| Correction: Q64.0.1 Penile epispadias. | ||
| Correction: Q64.0.2 Total epispadias. | ||
| Q64.1 | Bladder exstrophy | |
| Q64.2 | Congenital posterior urethral valves | |
| Q64.3 | Other types of atresia and stenosis of the urethra and bladder neck | |
| Q64.5 | Congenital absence of the bladder and urethra | |
| Q64.7 | Other congenital anomalies of the bladder and urethra | |
| Q64.8 | Other specified congenital anomalies of the urinary system | |
| Q64.9 | Congenital anomaly of the urinary system, unspecified | |
| Q89.7 | Multiple congenital anomalies not elsewhere classified | |
| Q89.8 | Other specified congenital anomalies | |
| Q89.9 | Congenital anomaly, unspecified | |
| Q92.7 | Triploidy and polyploidy | |
| Q95.3 | Balanced sexual/autosomal rearrangements in a normal individual | |
| Q98.0 | Klinefelter syndrome, karyotype 47,XXY | |
| Q98.1 | Klinefelter syndrome, male with more than two X chromosomes | |
| Q98.2 | Klinefelter syndrome, man with 46,XX karyotype | |
| Q98.3 | Another man with a 46,XX karyotype | |
| Q98.4 | Klinefelter's syndrome, unspecified | |
| Q98.5 | Karyotype 47,XYU | |
| Q98.6 | A man with structurally altered sex chromosomes | |
| Q98.7 | Male with mosaic sex chromosomes | |
| Q98.8 | Other specified sex chromosome abnormalities, male phenotype | |
| Q98.9 | Sex chromosome abnormality, male phenotype, unspecified | |
| Q99 | Other chromosomal abnormalities not elsewhere classified | |
| Q99.0 | Mosaic [chimera] 46,ХХ/46,ХY | |
| Q99.1 | 46.XX true hermaphrodite | |
| Q99.2 | Fragile X chromosome | |
| Q99.8 | Other specified chromosomal abnormalities | |
| Q99.9 | Chromosomal abnormality, unspecified | |
| R10.2 | Pain in the pelvis and perineum | |
| R30.9 | Painful urination, unspecified | |
| R32 | Urinary incontinence, unspecified | |
| R33 | Urinary retention | |
| R36 | Discharge from the urethra | |
| R39 | Other symptoms and signs related to the urinary system | |
| R39.1 | Other difficulties related to urination | |
| R39.8 | Other and unspecified symptoms and signs related to the urinary system | |
| R44.8 | Other and unspecified symptoms and signs relating to general sensations and perceptions | |
| R45 | Symptoms and signs related to emotional state | |
| R45.0 | Nervousness | |
| R45.1 | Anxiety and agitation | |
| R45.2 | State of anxiety due to failures and misfortunes | |
| R45.3 | Demoralization and apathy | |
| R45.4 | Irritability and anger | |
| R45.7 | State of emotional shock and stress, unspecified | |
| R45.8 | Other symptoms and signs related to emotional state | |
| R46 | Symptoms and signs related to appearance and behavior | |
| R52.1 | Constant, unrelieved pain | |
| R52.2 | Other constant pain | |
| R52.9 | Unspecified pain | |
| R53 | Malaise and fatigue | |
| R54 | Old age | Controversial diagnosis. |
| R62 | Lack of expected normal physiological development | |
| R62.0 | Delayed developmental milestones | |
| R62.8 | Other delays in expected normal physiological development | |
| R62.9 | Lack of expected normal physiological development, unspecified | |
| S30.2 | Bruise of the external genitalia | |
| S31.2 | Open wound of the penis | |
| S31.3 | Open wound of the scrotum and testicles | |
| S31.5 | Open wound of other and unspecified external genitalia | |
| S38.0 | Crushing of the external genitalia | |
| S38.2 | Traumatic amputation of the external genitalia | |
| T19 | Foreign body in the genitourinary tract | |
| T19.0 | Foreign body in the urethra | |
| T19.8 | Foreign body in another or several parts of the genitourinary tract | |
| T19.9 | Foreign body in an unspecified part of the genitourinary tract | |
| Z84.2 | Family history of other diseases of the genitourinary system | |
| Z87.4 | Personal history of diseases of the genitourinary system | |
| Z90.7 | Acquired absence of sexual organ(s) | |
| Z99.8 | Dependence on other auxiliary mechanisms and devices | |
This summary table shows those symptoms, syndromes and nosological forms according to ICD10 that a practicing specialist in the field of andrology encounters in real practice. At the same time, in our opinion, some of them no longer correspond to the modern understanding of pathophysiological processes and require terminological and classification clarification (right column).
Naturally, the above classification needs to be supplemented and refined, and fundamental amendments be made taking into account interdisciplinary interaction. With our publication, we would like to provoke a discussion among specialists in the field of andrology, the main goal of which would be to create a single, integral classification of andrological diseases. This is undoubtedly valuable and convenient for medical practitioners, health authorities, scientific research, and insurance companies. We will be grateful for your comments and remarks, which can be sent to the email address
Key words: ICD-10, andrological diseases, clinical and statistical classification.
Keywords : ICD-10, andrology diseases, clinical and statistical classification.
Literature
- International classification of diseases and related health problems. WHO, 1992
- Order of the Ministry of Health of the Russian Federation dated May 27, 1997 No. 170 (as amended on January 12, 1998) “On the transition of health authorities and institutions of the Russian Federation to the international statistical classification of diseases and health-related problems of the X revision.”
- Sivkov A.V., Kakorina E.P., Keshishev N.G. Unified clinical and statistical classification of urological diagnoses // Materials of the XI Congress of Urologists of Russia. M. 2007. pp. 598-599.
- Unified clinical and statistical classification of urolithiasis / Apolikhin O.I., Dzeranov N.K., Sivkov A.V., Kakorina E.P., Keshishev N.G. // Urology. 2008. No. 6. P. 3-6.
- On the creation of a unified clinical and statistical classification of oncological urological diseases using the example of kidney tumors / Apolikhin O.I., Sivkov A.V., Chernyshev Yu.V., Kakorina E.P., Keshishev N.G., Zhernov A.A. // Experimental and clinical urology. 2010. No. 1. pp. 23-28.
| Attached file | Size |
| Article in PDF format | 414.14 kb |