Unidox Solutab
Use during pregnancy and breastfeeding
Doxycycline penetrates the blood-placental barrier.
Tetracyclines have an adverse effect on the fetus (slowing osteogenesis) and on the formation of tooth enamel (irreversible discoloration, hypoplasia). Due to this, as well as the increased risk of liver damage in the mother, tetracyclines are not used during pregnancy, except in cases where the drug is the only remedy for the treatment or prevention of particularly dangerous and severe infections (Rocky Mountain spotted fever, inhalation exposure to Bacillus anthracis and others). Before prescribing doxycycline to women of childbearing age, pregnancy should first be excluded.
Doxycycline passes into breast milk. Due to its adverse effects on the fetus, doxycycline, like other tetracyclines, should not be used during breastfeeding. If the prescription of tetracyclines is necessary, breastfeeding should be discontinued.
Use for liver dysfunction
Contraindicated in severe liver dysfunction.
For patients with impaired liver function, Unidox Solutab® is prescribed only if treatment with other drugs is impossible.
In case of liver dysfunction, it is necessary to reduce the dose of the drug.
Use for renal impairment
Contraindicated in severe renal impairment.
Use in children
Contraindicated in children under 8 years of age.
special instructions
There is a possibility of cross-resistance and hypersensitivity with other tetracycline drugs.
Tetracyclines may increase prothrombin time; the use of tetracyclines in patients with coagulopathies should be carefully monitored.
The anti-anabolic effect of tetracyclines can lead to an increase in the level of residual urea nitrogen in the blood. As a rule, this is not significant for patients with normal renal function. However, in patients with renal failure, an increase in azotemia may occur. The use of tetracyclines in patients with impaired renal function requires medical supervision.
With long-term use of the drug, periodic monitoring of laboratory blood parameters, liver and kidney function is required.
Due to the possible development of photodermatitis, it is necessary to limit insolation during treatment and for 4-5 days after it.
When using the drug, both while taking it and 2-3 weeks after stopping treatment, diarrhea caused by Clostridium dificile may develop. In mild cases, it is sufficient to discontinue treatment and use ion exchange resins (colestyramine, colestipol); in severe cases, replacement of loss of fluid, electrolytes and protein, and the appointment of vancomycin or metronidazole are indicated.
Do not use medications that inhibit intestinal motility.
Long-term use of the drug can cause dysbacteriosis and, as a result, the development of hypovitaminosis (especially B vitamins).
To prevent dyspeptic symptoms, it is recommended to take the drug with meals.
To avoid the development of esophagitis or esophageal ulcers, it is necessary to take the drug with plenty of water and avoid using the drug immediately before bedtime.
Impact on the ability to drive vehicles and operate machinery
The effect on the ability to drive vehicles, machines and mechanisms is unknown. If you develop dizziness, blurred vision or double vision, driving vehicles or using machinery is not recommended.
Bacterial vaginosis
The normal microflora of the vagina of women of reproductive age is one of the indicators of health, as it plays a large role in maintaining the microecological status. The interaction between representatives of normal microflora and vaginal epithelial cells takes place at the cellular and molecular levels and is constantly monitored by a number of systems of the macroorganism. The result of this interaction is the creation and maintenance of high colonization resistance of the vaginal epithelium to the introduction of pathogenic and opportunistic microorganisms [1–6].
Bacterial vaginosis is separated from the category of nonspecific vaginitis into a separate nosological form. It includes pathological conditions in the vagina, accompanied by disturbances in the qualitative and quantitative composition of normal microflora and not associated with bacterial sexually transmitted infections (STIs), fungi or protozoa.
According to modern concepts, bacterial vaginosis is an infectious non-inflammatory syndrome of polymicrobial etiology, which is associated with dysbiosis of the vaginal biotope. Disruption of the microecology of the vagina in bacterial vaginosis is characterized by a sharp decrease in the number or absence of lactobacilli producing hydrogen peroxide and an increase in the number of Gardnerella vaginalis, gram-negative anaerobic bacteria (Bacteroides spp., Mobiluncus spp., Fusobacterium spp., Peptostreptoicoccus spp., M. hominis, U. urealyticum ).
Gardnerella, like lactobacilli, have a pronounced ability to adhere to the surface of vaginal epithelial cells. Gardnerella vaginalis can produce toxic bioproducts, which include mucolytic enzymes and hemolysin, which is a leukotoxic factor. Hemolysin, acting on erythrocytes, causes the formation of numerous pores in the erythrocyte membrane, and also affects leukocytes, causing their structural and functional disorders. This explains the absence of significant leukocyte infiltration, i.e., leukocyte activity in the presence of Gardnerella vaginalis.
Representatives of the Mycoplasmataceae family - Mycoplasma hominis and Ureaplasma urealyticum - play a certain role in the pathogenesis of bacterial vaginosis. 70% of the isolated Ureaplasma urealyticum strains belong to the Parvo biovar, 23.3% to the T960 biovar. Most of the patients from whom Ureaplasma urealyticum, belonging to the biovar Parvo, was isolated, had in the past early onset of sexual activity, frequent changes of sexual partners, the presence of several sexual partners at the same time, a large number of pregnancies and abortions, as well as concomitant gynecological diseases and STIs.
When examining sexual partners of patients with bacterial vaginosis, morphotypes of microorganisms associated with bacterial vaginosis were detected in 25% of men, while clinical manifestations (balanoposthitis) were observed only in 3.1% [2].
As a result of a submicroscopic study of vaginal exudate from patients with bacterial vaginosis, the structural and functional characteristics of microbial cells involved in interaction with each other were determined, while the cells of the host body are represented predominantly by squamous epithelium. The gram variability of Gardnerella vaginalis has been established, regardless of the nature of microbial associations. Analysis of electron diffraction patterns demonstrated that the structural organization of Gardnerella vaginalis adhered to the surface of epithelial cells did not depend on the combination of bacterial vaginosis with other infectious agents.
When bacterial vaginosis was combined with Chlamydia trachomatis, Mycoplasma hominis, Ureaplasma urealyticum, a significant number of microorganisms in the intercellular space and bacteria morphologically identical to mycoplasmas were observed [2].
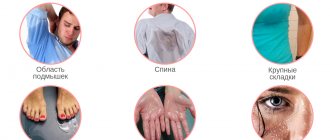
Patients complain of copious white or gray discharge from the genital tract, often with an unpleasant odor, especially after sexual intercourse or during menstruation. During a long-term process, the discharge acquires a yellowish-green color, becomes thicker, often resembles a cheesy mass, and has the property of foaming; slightly viscous and sticky, they are evenly distributed over the walls of the vagina. The amount of leucorrhoea varies from moderate to very heavy, averaging 20 ml per day. Complaints of itching and dysuric disorders are rare: they may be completely absent or appear periodically. These symptoms are found in 16–23% of patients with disorders of the vaginal microflora. A characteristic sign of bacterial vaginosis is the absence of inflammation of the vaginal walls. Often, women with bacterial vaginosis complain of heavy menstrual bleeding and pain in the lower abdomen. At the same time, some patients do not have any subjective sensations.
Diagnosis of bacterial vaginosis is based on medical history, assessment of subjective and objective symptoms of the disease, results of laboratory tests - microscopic examination of the material, amine test and pH of vaginal exudate [3].
When collecting anamnesis, they find out:
- past treatment with antibiotics, cytostatics, corticosteroids, antiviral and antifungal drugs;
- the presence of gynecological, endocrine diseases, diseases of the gastrointestinal tract, etc.;
- compliance with the rules of personal and sexual hygiene;
- data on sexual partners, the practice of sexual contacts and the condition of the genitourinary system of sexual partners;
- use and methods of contraception.
The diagnosis of bacterial vaginosis is established based on the presence of three criteria from the following:
- homogeneous discharge of a whitish-gray color, evenly adhered to the mucous membrane of the vulva and vagina, having an unpleasant odor;
- pH of vaginal exudate > 4.5;
- a positive result of the amine test (the appearance of a fishy odor when mixing vaginal discharge with a 10% KOH solution in equal proportions on a glass slide);
- changes in the microcenosis of the vagina, revealed by microscopic examination of vaginal exudate.
The patient should be examined no earlier than 72 hours after the last sexual intercourse (without using a condom); it is also not performed during menstruation. For 3 weeks before the examination, the woman should not receive treatment with systemic and local antibacterial drugs [3].
Microscopic examination of a native and Gram-stained vaginal smear reveals the following signs:
- massive, less often - a large number of microbial cells with a predominance of morphotypes of anaerobes and Gardnerella vaginalis;
- complete absence or single presence of lactobacilli morphotypes;
- the vaginal epithelium is represented by cells of the superficial layers, intermediate cells are rarely found, often the so-called “key” cells (epithelial cells of the vagina, on the surface of which gram-variable coccorbacillary microflora is adhered);
- absence of leukocyte reaction (1/3 of women with bacterial vaginosis have no leukocyte reaction).
The assessment of the total microbial contamination of vaginal discharge is carried out using a 4-point system - according to the number of microbial cells detected in one field of view during immersion microscopy:
1 point (+) - up to 10 microbial cells in the field of view, a small number of them (scanty growth);
2 points (++) - from 11 to 100 microbial cells in the field of view, their number is moderate;
3 points (+++) - from 100 to 1000 microbial cells in the field of view, a large number of them;
4 points (++++) - more than 1000 microbial cells in the field of view, a massive number of them.
Since Gardnerella vaginalis can be detected in healthy women, culture and PCR diagnostics are not performed to identify Gardnerella vaginalis. A cultural study is carried out if there are indications to determine the species and quantitative composition of the vaginal microcenosis and exclude pathogens of STIs.
A cultural examination may reveal changes characteristic of bacterial vaginosis: total microbial contamination exceeds 109 CFU/ml; when using only aerobic cultivation conditions, there is no growth of microorganisms or growth of accompanying opportunistic microorganisms is observed (usually in a small titer); polymicrobial nature of the microflora with an absolute predominance of obligate anaerobic species and Gardnerella vaginalis; lack of growth of lactobacilli or a sharp decrease in their titer (< 104 CFU/ml).
Based on the studies conducted, it is currently recommended that the patient management plan include:
- analysis of subjective and objective manifestations of the disease, obstetric, gynecological and sexual history data with an emphasis on previous or concomitant diseases of the urogenital system;
- comprehensive assessment of vaginal microbiocenosis, including identification of STI pathogens;
- identification of Mycoplasma hominis, Ureaplasma urealyticum by cultural method with quantitative assessment of pathogens;
- determination of the biological identity of Ureaplasma urealyticum to decide on the choice of tactics for further management of the patient;
- it is advisable to involve specialists in related disciplines (gynecologists, urologists) in the presence of concomitant diseases of the urogenital system;
- clinical and microbiological examination of sexual partners (preventive treatment of sexual partners of patients with bacterial vaginosis is currently considered inappropriate).
The goal of treatment is to reduce the severity of clinical symptoms, normalize laboratory parameters, and prevent the development of possible complications during pregnancy, as well as in the postpartum period and when performing invasive gynecological procedures.
Normal laboratory results are as follows.
- On microscopic examination:
- moderate or large number of microbial cells with a predominance of lactobacilli morphotypes;
- the vaginal epithelium is represented by cells of the superficial layers, cells of the intermediate layer are less common, and there are no “key” cells;
- leukocyte reaction is absent or weakly expressed - single leukocytes in the field of view.
- When examining the pH of vaginal exudate: 3.8–4.5.
- Amine test: negative (no fishy odor when mixing vaginal discharge with 10% KOH solution in equal proportions on a glass slide)
- During microbiological examination:
- total microbial contamination 106–108 CFU/ml;
- absolute predominance of lactobacilli;
- opportunistic microorganisms in low titer (104 CFU/ml) or absent.
Treatment
The main direction of therapy is the use of local or systemic antibacterial drugs with an antianaerobic effect.
The use of clindamycin is indicated. The drug is a 7-deoxy derivative of lincomycin, inhibits protein synthesis in microorganisms, has a bacteriostatic or bactericidal effect depending on the concentration in the macroorganism and the sensitivity of the microorganism. The drug is effective against gram-positive microorganisms (staphylococci, streptococci, pneumococci, diphtheria bacillus), gardnerella, mycoplasma. Resistance of microorganisms to clindamycin develops slowly. When taken orally, clindamycin is absorbed better than lincomycin. After intramuscular administration, the maximum concentration in the blood is observed after 2–2.5 hours. The drug penetrates well into body fluids and tissues and is excreted in urine and bile. If renal and liver function are impaired, the elimination of clindamycin slows down. For bacterial vaginosis, one of the dosage forms of clindamycin can be prescribed: 2% cream 5 g in an applicator (single dose) intravaginally 1 time per day (at night) for 6 days; ovulation 100 mg intravaginally at night for 3 days; capsules 300 mg orally 2 times a day for 7 days.
Clindamycin is approved for use in pregnant women in the form of 2% cream 5 g (single dose) intravaginally 2 times a day for 5 days.
Metronidazole can also be prescribed for bacterial vaginosis. It has a wide spectrum of action against protozoa, suppresses the development of Trichomonas vaginalis, Entamoeba histolytica and Giardia. The drug is highly effective against anaerobic bacteria. Metronidazole is well absorbed when taken orally, penetrates organs and tissues, passes through the placenta and blood-brain barrier, and accumulates in the liver. The half-life of the drug is 8–10 hours and is completely eliminated from the body 1–2 days after administration. Metronidazole is mainly excreted in the urine unchanged and in the form of metabolites, partially in the feces. When using metronidazole, loss of appetite, dryness and unpleasant taste in the mouth, nausea, vomiting, diarrhea, headache, urticaria, and itching may occur. These phenomena disappear after the end of treatment or discontinuation of the drug. Possible leukopenia. The drug is contraindicated during pregnancy and breastfeeding, hematopoietic disorders, and acute diseases of the central nervous system. To avoid the development of severe adverse reactions, patients should be warned not to consume alcohol and products containing it both during metronidazole therapy and for 24 hours after its end. For bacterial vaginosis, one of the following treatment regimens using metronidazole can be used:
- gel 0.75% 5 g (single dose) intravaginally (at night) for 5 days;
- tablets 500 mg orally 2 times a day for 7 days;
- tablets 2 g orally once.
Other metronidazole derivatives are also used:
- tinidazole (Tinidazole-Acri, Verotinidazole), tablets 2 g orally once;
- ornidazole (Ornidazole-Vero), tablets 500 mg orally 2 times a day for 5 days.
Previously, a comparative study of the effectiveness of topical probiotics was conducted. Since there were no significant differences in treatment results in patients who received and did not receive these drugs, they are currently not recommended for the treatment of bacterial vaginosis [3].
When bacterial vaginosis is combined with an STI, antibacterial drugs are simultaneously used in accordance with the nosological form of the disease [2]. For uncomplicated gonorrheal infection, ceftriaxone is prescribed intramuscularly in a single dose of 250 mg (the drug is a third-generation cephalosporin antibiotic; after intramuscular administration, the peak concentration in the blood is observed after 1.5 hours; it is excreted slowly from the body). If chlamydia and/or mycoplasmas are detected, the use of antibiotics - macrolides, tetracyclines - is indicated.
The high therapeutic effect and good tolerability have contributed to the significant spread of the use of macrolides. Their antimicrobial effect is due to disruption of protein synthesis in the ribosomes of the microbial cell. As a rule, macrolides have a bacteriostatic effect, but in high concentrations they can also cause a bactericidal effect. In addition to their antibacterial effect, macrolides have moderate immunomodulatory and anti-inflammatory activity. A representative of the third generation of macrolides is josamycin (Vilprafen). This antibiotic is quickly absorbed from the gastrointestinal tract (GIT), penetrates well through biological membranes and accumulates in tissues. The maximum concentration is reached 1–2 hours after administration. 45 minutes after taking a dose of 1 g, the average concentration of josamycin in plasma is 2.41 mg/l; binding to plasma proteins does not exceed 15%. Taking the drug at intervals of 12 hours ensures that the effective concentration of josamycin in tissues is maintained throughout the day. An equilibrium state is achieved after 2–4 days of regular use. The concentration of josamycin in human polymorphonuclear leukocytes, monocytes and alveolar macrophages is approximately 20 times higher than in other cells of the body. The drug is biotransformed in the liver to less active metabolites. Josamycin is excreted mainly in bile, but excretion in urine is less than 20%. The drug is prescribed 500 mg 2 times a day for 10 days.
From the group of tetracyclines, doxycycline is the most effective for STIs; In recent years, Unidox Solutab has been widely used. The drug differs from Doxycycline hydrochloride in its neutral reaction, has less irritating effect on the mucous membrane of the digestive tract and has improved antimicrobial and pharmacokinetic properties. Unidox Solutab blocks ribosomal polymerase and inhibits protein synthesis in microorganisms.
Unidox Solutab tablets have controlled solubility. They can not only be taken orally whole or in parts, but also quickly prepared from them into a syrup, suspension (dissolving the tablet in 20 ml of water) or solution (dissolving the tablet in 100 ml of water), which have a pleasant taste. The tablets dissolve in water within 5–10 s, in biological fluids within 1 min, turning into a uniform suspension.
Unidox Solutab is completely absorbed. The bioavailability of the drug is 95%. 2 hours after dosing (200 mg on the first day and 100 mg on subsequent days), serum levels range from 1.5 to 3 mg/ml. The drug is 80–90% bound to plasma proteins, penetrates well into tissues, and accumulates in the reticuloendothelial system. The half-life is 16–18 hours, after repeated doses it is 22–23 hours.
Previously, advantages were noted in the absence of side effects such as esophagitis, which occur when using conventional forms of Doxycycline hydrochloride, the ability to accumulate in high concentrations in the reproductive organs and actively influence the infectious agent.
Unidox Solutab is prescribed at a dose of 200 mg for 10 days.
Laboratory monitoring of the effectiveness of therapy should be carried out immediately after the end of etiotropic treatment: by microscopy of vaginal smears stained with Gram, it is necessary to ascertain the degree of eradication of microorganisms associated with bacterial vaginosis; when culturing vaginal discharge, detect cases of colonization with facultative anaerobic opportunistic microorganisms.
When bacterial vaginosis is combined with urogenital chlamydia and/or mycoplasmosis, laboratory monitoring must be repeated 3 weeks after the end of therapy.
For questions regarding literature, please contact the editor.
I. V. Khamaganova, Doctor of Medical Sciences, Professor of Russian State Medical University, Moscow
Instructions for use UNIDOX SOLUTAB®
It is preferable to take the drug with meals (to prevent dyspeptic symptoms). The tablets are dissolved in a small amount of water (about 20 ml) to obtain a suspension. The tablets can also be swallowed whole, split into pieces, or chewed with water. It is preferable to take the tablets while sitting or standing, which reduces the likelihood of developing esophagitis and esophageal ulcers. The drug should not be taken immediately before bedtime.
Usually the duration of treatment is 5-10 days.
Adults and children over 12 years old
on the first day of treatment, 200 mg/day is prescribed in 1 or 2 doses, on subsequent days of treatment - 100 mg/day daily.
In case of severe infections,
200 mg/day is prescribed daily for the entire treatment period.
For infection caused by Streptococcus pyogenes,
The duration of treatment is at least 10 days.
For uncomplicated gonorrhea
(with the exception of anorectal infections in men),
adults
are prescribed 100 mg 2 times a day until complete cure (on average within 7 days), or 600 mg are prescribed for one day - 300 mg in 2 doses (the second dose after 1 hour after the first one).
With primary syphilis
prescribed 100 mg 2 times/day for 14 days, for
secondary syphilis
- 100 mg 2 times/day for 28 days.
For uncomplicated urogenital infections caused by Chlamydia trachomatis, cervicitis, non-gonococcal urethritis caused by Ureaplasma urealiticum
, prescribed 100 mg 2 times a day for 7 days.
For acne
Prescribed 50 mg/day, course of treatment - 6-12 weeks.
For the prevention of malaria
prescribed 100 mg 1 time/day 1-2 days before the trip, then daily during the trip and for 4 weeks after returning;
children over 8 years old
- 2 mg/kg 1 time/day.
To prevent traveler's diarrhea
- 200 mg on the first day of the trip in 1 or 2 doses, then - 100 mg 1 time / day during the entire stay in the region (no more than 3 weeks).
For the treatment of leptospirosis
- 100 mg orally 2 times a day for 7 days;
for the prevention of leptospirosis
- 200 mg 1 time per week during stay in a disadvantaged area and 200 mg - after departure. There are no data on the use of the drug for prophylactic purposes longer than 21 days.
To prevent infections during medical abortion
Prescribe 100 mg 1 hour before and 200 mg after the intervention.
Maximum daily doses for adults
- up to 300 mg/day or up to 600 mg/day for 5 days for
severe gonococcal infections
.
For children over 8 years old weighing more than 50 kg -
up to 200 mg, for
children 8-12 years old weighing less than 50 kg
- 4 mg/kg daily throughout treatment.
In case of renal (creatinine clearance less than 60 ml/min) and/or liver failure
a reduction in the daily dose of doxycycline is required, since this results in a gradual accumulation of it in the body (risk of hepatotoxicity).