Lichen planus is a chronic disease, prone to relapse, which is accompanied by itchy skin and inflammatory rashes in the form of purple papules.
This disease often affects the mucous membranes of the mouth or genitals. According to WHO, lichen planus accounts for about 2.5% of all dermatological diseases and 15% of diseases of the oral mucosa.
The complex of diseases that make up lichen planus includes two syndromes: Little-Lassuere syndrome, including non-scarring alopecia of the armpits and pubis and scarring alopecia of the scalp, as well as Grinshpan-Potekaev syndrome, including diabetes, hypertension and an erosive-ulcerative form of the dermatological disease.
The exact cause of the pathology is unknown. There are neurogenic, viral, bacterial, autoimmune and toxic-allergic theories of the nature of the disease.
There are a number of factors that increase the likelihood of developing lichen planus, namely:
- genetic predisposition;
- nervous system disorders;
- failure of immunity;
- allergic (lichenoid) reactions to chemical or food components;
- chronic infections in the body;
- metabolic disorders;
- stress;
- injuries to the skin and oral mucosa (presence of sharp edges of teeth, poorly made dentures);
- changes in the microelement composition of saliva due to the presence of dentures made of different metals in the oral cavity;
- aggressive effect of certain medications;
- age after 40 years, etc.
Treatment of this disease is complex and requires an individual approach. Based on the test results, the doctor selects a regimen and dosage of drugs, usually of local action, which reduce the symptoms of the pathology and prevent its reappearance.
What is lichen planus?
Lichen planus is an inflammatory dermatosis with various clinical manifestations. The pathological process involves skin tissue and its appendages (nails and hair), as well as mucous membranes. Usually the disease affects representatives of both sexes of middle and older age, but sometimes it is observed in children.
In modern medicine, it is believed that lichen planus is a delayed-type hypersensitivity reaction in response to the action of a neoantigen of epidermal (skin) origin, since the autoimmune aspect has a significant place in the nature of the disease. Cytokines released by immune cells provoke cytotoxic mechanisms and trigger cell death (apoptosis).
Lichen planus is characterized by three stages:
- progressive, which is accompanied by the active manifestation of symptoms of the pathology: the formation of papules and itching;
- stationary with a gradual decrease in signs of the disease: pallor, flattening of papules and subsidence of itching;
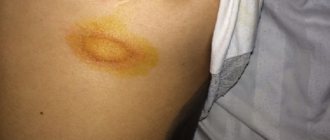
- regressive, which is characterized by regression of papules, the formation of brown spots and areas of atrophy at the site of their localization, and the disappearance of itching.
How to identify lichen planus?
The external manifestation of lichen planus is caused by the processes of damage and regeneration of skin cells and mucous membranes.
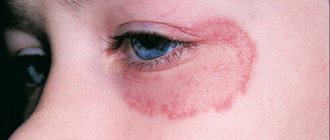
The following symptoms are characteristic of lichen planus:
- the presence of polygonal, flat-topped, colored papules 1-2 mm in diameter;
- grouping of papules with the formation of rings or lines;
- the formation of large plaques from papules (Wilham stack), along the periphery of which isolated small papules are located;
- symmetrical localization on the skin (wrist joints, forearms, legs, genitals, etc.);
- the appearance of neoplasms on the inside of the cheeks, gums, tongue, red border of the lips or genitals when the mucous membrane is damaged;
- thinning, brittleness, clouding of the nail plates;
- pronounced itching;
- rapid appearance of papules due to skin injuries (Koebner phenomenon);
- formation of secondary hyperpigmentation in place of the disappeared papule.
Doctors classify the main morphological forms of lichen planus as:
- typical, most common (45% of cases) form of pathology, accompanied by the formation of pearl-colored papules with a specific coating;
- hyperkeratotic or verrucous, characterized by the formation of hard red, gray-yellow or dark red infiltrated plaques on the front of the leg or upper limbs;
- exudative-hyperemic (25% of cases), causing the formation of edematous papules;
- erosive-ulcerative, often resulting from exudative-hyperemic lichen and accompanied by the formation of small ulcers or areas of erosion on the feet or in the interdigital space, etc.
Also in some cases, rare bullous, atypical and pigmented forms of the disease are observed.
The appearance of the first signs of the disease requires immediate medical response. Lack of timely treatment can lead to rapid progression of the pathology and a significant weakening of the body's defenses. What tests are done for lichen planus?
Typically, the diagnosis of lichen planus includes:
- blood test (general and biochemical);
- hormonal profile;
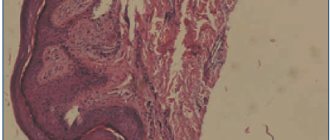
- scraping for histological and cytomorphological examination (affected tissues are susceptible to hyperkeratosis, degeneration of the epidermis, the presence of infiltrate, etc.);
- skin biopsy;
- consultation with a dentist and other related specialists (endocrinologist, gastroenterologist).
When visiting a doctor, it is extremely important to provide him with a list of medications taken, episodes of recent dental treatment, vaccination or viral disease. History data will greatly help in making the correct diagnosis and determining the nature of the origin of lichen planus.
Introduction
Lichen planus (LP) is a subacute or chronic inflammatory dermatosis affecting the skin, mucous membranes of the oral cavity, esophagus, pharynx, conjunctiva of the eye and skin appendages [1].
Its population frequency is estimated at 0.4–1.9% and has more than doubled in recent years [2]. The disease is considered an important interdisciplinary problem, in which dermatologists, dentists, gynecologists and doctors of a number of other specialties take part [3]. LP most often affects adults over 40 years of age, mainly women. The cause is unclear, and the mechanism of development is associated with autoimmune destruction by T cells of basal keratinocytes altered by viruses, medications, or exposure to other allergens. In this case, the main role is played by the activation of the Th1 immune response, leading to apoptosis of basal keratinocytes [4]. Antigen-presenting Langerhans cells, the number of which significantly increases in the early elements of LP, interacting with T-lymphocytes due to the activation of aberrant production of proinflammatory cytokines (INF-γ, TNF-α, IL-Iα, IL-6, IL-8, AS/Apo- 1 and Bcl-2, CXCLI0), convert them into cytotoxic for keratinocytes, causing degeneration and destruction of keratinocytes [5]. There are typical and atypical clinical forms of LP of the skin and mucous membranes. Typical LLP is characterized by itchy pink-purple papules 1–5 mm in diameter, polygonal in shape with clear borders, with a smooth shiny surface and a central umbilical depression. On the surface of the papules, a lacy network of whitish lines and dots forms a Wakeham grid. The rash is located symmetrically, usually in the area of the folds of the wrist joints and forearms, the anterior surface of the ankle joints and legs, in the area of the sacrum, neck, buttocks, and genitals [6]. Among the atypical forms of LP, atrophic LP is distinguished; LP, characterized by an atrophic plaque with a whitish-pearlescent tint; pigmented, manifested by dark brown spots; follicular, manifested by follicular elements; bullous, etc. [7]. Among the OLP of the oral mucosa (OCLP), six forms are distinguished: erosive, bullous, infiltrative, atrophic, atypical, reticular [8]. They can pass from one to another and often occur against the background of suppression of the cellular component of immunity.
The course of atypical LP is less favorable than typical LP. It often persists for many years and even decades, primarily in the hypertrophic, atrophic, follicular and erosive forms of CPLP [9]. On average, CPLP exists for about 5 years and transforms into cancer with a statistically significant frequency [10]. Due to the uncertainty of its etiology, therapy for LP is not effective enough. Standards of therapy for LP include external, intralesional and systemic corticosteroids, retinoids, and in cases that are difficult to treat - cyclosporine. In all cases, treatment of patients with LLP is carried out comprehensively, taking into account the form, stage of the disease, the prevalence of rashes and their localization, the depth of damage to the skin and mucous membranes, the involvement of skin appendages, hair and nails in the pathological process, secondary changes (cicatricial alopecia, skin atrophy), severity itching, as well as the age of the patient.
Methods of radiation therapy for LP
Since the late 1970s. A major contribution to the treatment of patients with LP was made by PUVA therapy, based on the ingestion of the photosensitizer 8-methoxypsoralen (8-MOP) and subsequent irradiation of the entire body surface with UVA in the range of 320–400 nm. For the first time, the resolution of rashes shortly after 6 sessions of irradiation of patients with widespread LP was reported by W. Brenner et al. [11], and J. Ortone et al. these data were confirmed [12]. And although PUVA therapy provides not only local, but also systemic effects, as evidenced by improvement in the pathological process both on the non-irradiated side of the body and in the oral cavity [13], it later turned out that this method is not so effective (50–90% ), especially with widespread skin and severe erosive oral lesions, often gives relapses and to achieve a full clinical effect requires large cumulative doses of UVR (7.2–23.9 J/cm2), significantly increasing the carcinogenic danger [14]. The negative side of PUVA therapy for LP is the long-term persistence of post-inflammatory hyperpigmentation, which is not always cosmetically acceptable [15], and the exacerbation of actinic LP under the influence of PUVA therapy [13]. All this significantly limited the use of PUVA therapy for LP.
In the 1980s PUVA baths began to be used for LLP. Although this method required larger total cumulative doses of UVR (10.1–32.9 J cm2) than traditional PUVA therapy, it was considered safer [16], but did not prevent the development of early relapses of the disease. Therefore, for resistant forms of LLP of the oral mucosa, primarily erosive ones, local PUVA therapy has been proposed [17], based on a combination of oral psoralen and irradiation of the oral mucosa with a device with a filter, usually used to seal dental fillings. However, the method required great caution and, due to possible burns, due to the difficulty of ensuring homogeneity of irradiation of the oral cavity for the required time, it was not widely known [18].
Currently, for non-acute forms of widespread LP, narrow-band UVB therapy is used [19], the effectiveness of which is based on the induction of T-cell apoptosis and, according to some authors, is higher [20], according to others, it is lower than PUVA therapy, but superior its tolerability and safety [21]. Narrowband UVB therapy sessions are carried out 5–6 times a week [19]. For generalized LP, UV-A1 therapy is performed [22], as well as combined narrow-band medium-wave (311 nm) and broadband long-wave (320–400 nm) phototherapy [23]. For LP of the oral cavity, including hyperkeratotic, a combination of PUVA therapy with retinoids (re-PUVA therapy) is used [24], and for dysplastic LP, laser and surgical removal is used (less commonly, applications of ointment with bleomycin, vitamin A, neotigazone) [25 ]. However, these methods are not effective enough and have numerous side effects.
In severe, therapy-resistant cases of LP and CLPPR, a number of authors have successfully used infusions of retuximab (an anti-CD-20 monoclonal antibody) [26], in CLPPR - etanercept (a monoclonal antibody to TNF-a), but the high cost and the need for long-term treatments significantly limit their use [27], in addition, these biological drugs increase the risk of secondary infections, heart failure and malignancy [28]. Due to the insufficient effectiveness of drug therapy for LP in complex treatment, along with LP, physiotherapy is widely used: UV irradiation, hyperbaric oxygenation, laser ablation, as well as inductothermy of the lumbar region, diadynamic currents, dosed focal vacuum [29]. In recent years, photodynamic therapy (PDT) with photosensitizers toluidine blue and 8-methoxypsoralen has been used for limited, treatment-resistant foci of LP and erosive foci of LPPR. The effectiveness of these drugs is associated with their ability to induce apoptosis of hyperproliferating inflammatory cells [30, 31]. The method is minimally invasive and, according to the authors, has a pronounced clinical effect without scar formation. F. Aghahosseini PDT, along with surgical excision, laser removal, applications of cytostatics (bleomycin) and systemic vitamin A preparations, is also used in the treatment of CLPR [30].
Laser ablation of CPLP lesions is carried out with the rays of a carbon dioxide laser, the action of which is based on its ability to destroy keratinocytes by protein denaturation, and a diode laser, the rays of which are also capable of destroying the underlying connective tissue and the inflammatory cells located there. However, the results of randomized trials of the effectiveness of this method compared with topical corticosteroids have been conflicting. Some researchers noted the advantage of medical lasers in erosive and atrophic CPLP [32, 33], while others noted the advantage of dexamethasone, triamsinolone and 0.05% clobetasol ointments [32]. Apparently. these lasers can be used as an alternative in refractory cases of CPLP [34].
A medical yttrium aluminum garnet laser emitting infrared light, used for thermal coagulation, ablation and evaporation. At a wavelength of 2.94 µM, it was effective in 2 women with CPLP refractory to topical corticosteroid therapy, who experienced rapid healing of the erosions with mild discomfort and minor bleeding during and after treatment. However, one of them relapsed after 15 months [35]. Complications with this laser include prolonged erythema, purpura, erosions, pigmentary disorders, and scar formation [36].
An excimer laser with a wavelength of 308 nm, emitting monochrome coherent light, producing immunomodulatory and photobiological effects similar to narrow-band UVB, stimulating apoptosis of inflammatory cells and suppressing the T-cell immune response [37], was used in two cases of refractory LPPR, which led to complete remission, but one patient relapsed after 4 weeks [38]. The treatment was well tolerated, with side effects including erythema, hyperpigmentation, blisters, erosions and pruritus. According to M. Trehan and CR Taylor, the use of this laser in the treatment of 5 (75%) patients led to significant improvement without relapse within 2 to 17 months, 2 patients – to improvement, in one there was no effect [39].
Data on the effectiveness of UV-B phototherapy for CPLP are based on one report of its use in 14 patients with erosive CPLP refractory to topical corticosteroids. Treatment after 8 weeks led to recovery in 9 cases, improvement in 5 cases, but relapse occurred in 4 patients. Side effects were noted in one case and were characterized by transient visual impairment [40]. In general, side effects of UVB include acute erythema, hyperpigmentation, decreased visual acuity, itching, and the development of erosions and blisters. In addition, a number of authors pointed out the carcinogenic risk when using UV-B from the oral mucosa [41].
PUVA therapy (syn.: photochemotherapy) with 8-methoxysoralen (8-MLP) and UV-A irradiation of lesions has an antiproliferative effect associated with suppression of DNA replication and apoptosis of inflammatory cells, as well as a suppressive effect on T cells and cytokine expression , which leads to suppression of the immune response. In addition, psoralens directly interact with cellular components and indirectly modify them through reactive oxygen species [42]. The method is effective in cases of LP that are refractory to topical corticosteroids, but the high frequency of side effects (nausea, eye symptoms, headache, itching, photosensitivity) [43] and the carcinogenic effect in a separate perspective [44] significantly limit its use.
Due to the unfavorable general and local effects of PUVA therapy [13], a number of researchers in the mid-1980s. came to the conclusion that it is possible to use extracorporeal photochemotherapy (EPCT) for advanced and generalized LP, which previously, in addition to T-cell lymphoma of the skin, was successfully used for LP-like graft-versus-host disease [45], as well as for a number of autoimmune diseases (rheumatoid arthritis). , systemic lupus erythematosus, pemphigus vulgaris, systemic scleroderma [46, 47]) diseases associated with disorders of T-lymphocytes [48]. The method is based on leukinpheresis, UVA irradiation of peripheral blood mononuclear cells isolated after oral administration of 8-MOP and their subsequent reinfusion to the patient [49].
The molecular basis of the effect of EPCT is the formation of thymidine dimers of DNA and RNA, leading to DNA damage and disruption of protein synthesis with the formation of an anticlonotypic immune response against pathogenic clones of T-cell populations based on the induction of apoptosis of pathogenic T-cells and activation of antigen-presenting cells [50, 51]. The method is effective for typical LP [52], however, data on its effectiveness in atypical forms of LP are based on isolated observations.
In this regard, of particular interest is the report on the pronounced and persistent clinical effect of phototherapy (3 sessions per month, 24 sessions per course) for treatment-resistant erosive LLP of the oral cavity, incl. combined with generalized LP of the skin [53], and with treatment-resistant (cyclosporine, azathioprine, PUVA therapy, acitretin, prednisolone) painful erosions of the soles, as well as scarring alopecia of the scalp (Graham–Little–Lassauer syndrome) [54]. The pathogenetic orientation of the method for LP is indicated by data from a number of researchers, according to which the effect of EPCT in this disease was accompanied by a significant decrease in the number of T-lymphocytes in the blood with a stable level of B-lymphocytes and natural killer cells [53]. The method is usually well tolerated. Side effects are rare and include hypotension, anemia, pruritus, and fever, which may occur during or after treatment [50].
Conclusion
Thus, LP is a very common disease, characterized by the presence of atypical forms of lesions of the skin and mucous membrane of the oral cavity, and erosive and ulcerative lesions are precancerous. In this regard, the use of EPCT in the complex treatment of lesions of LP and LPPR, incl. resistant to drug therapy, very promising and safe.
How dangerous is lichen planus?
Lack of treatment or a severe stage of the disease can lead to damage to the entire skin, severe discomfort and a significant decrease in immunity.
Lichen planus on the scalp can cause irreversible hair loss, and on the nail plates - nail deformation (thinning, splitting, rejection of the nail plate, even nail lysis).
A pathological lesion in the eye area can lead to blurred vision or narrowing of the tear duct.
The erosive form of the disease often leads to the formation of scars, and the result of lesions of the genital mucosa can be narrowing of the vagina or phimosis.
Also, some forms of the disease are prone to malignancy and degeneration into squamous cell skin cancer.
Recipes for external treatment
Anyone who knows first-hand what lichen ruber is would like to understand how to treat this disease at home. You can relieve inflammation, itching and tingling quickly enough using compresses, ointments, oils and decoctions.
Green doctor calendula: tinctures, decoctions
The anti-inflammatory, antiseptic and wound-healing properties of calendula make it a popular remedy for the fight against lichen erythematosus. This plant is used in the form of tinctures, ointments, and decoctions:
- Calendula flowers for compresses are filled with 70% alcohol and left for 2 weeks. After preparation, store for no more than 1 year. Instead of alcohol, you can use vodka.
- Calendula tincture is used to irrigate the oral cavity. To do this, dilute a teaspoon of tincture in a glass of boiled water. Sometimes foci of inflammation are cauterized with a cotton swab dipped in a clean tincture.
- You can use calendula for rubbing. For this, a decoction is prepared from dry leaves. 2 tbsp. l. plants are poured with 200 ml of boiling water and covered with a lid. Cover the top with a towel and express after a while. Treat the skin 2-3 times a day.
- In addition to compresses and rubdowns, calendula is used to prepare ointments. 10 g of inflorescences are ground into powder and mixed with 50 g of petroleum jelly. The resulting ointment is applied to the inflamed areas.
Herbal compresses
Despite the large number of folk remedies from calendula, other plants are also used as compresses for the treatment of lichen planus:
- Motherwort has long been famous for its medicinal properties. Teas and motherwort tinctures are used to relieve nervous tension and alleviate allergic manifestations. To prepare an infusion against red lichen, 1 tbsp. l. The plants are brewed in 1 cup of boiling water. Soak gauze in it and leave it on the inflamed areas.
- Take equal parts of dry chamomile, sage and calendula herbs, mix and add hot water. Let it brew and cool. A piece of gauze or cotton wool is moistened in the resulting decoction and applied to areas of hyperpigmentation.
- Another recipe will require 3 tbsp. l. mullein, 1 tbsp. l. willow and the same amount of celandine. Pour 1 liter of boiling water over the mixed herbs and leave for one hour. You can make compresses soaked in the broth several times a day. The resulting infusion can also be used to wipe the areas affected by lichen.
- Grind a few aloe leaves and put them in boiling water. After a day, moisten a gauze cloth in the resulting infusion and use it as a compress.
- Apple cider vinegar lotions can be applied to the area affected by lichen ruber 4-6 times a day. After a 10-minute procedure, irrigate the inflamed area with water. Instead of vinegar, you can use apple, viburnum or cranberry juice.
Herbal ointments
Effective in the treatment of ringworm ointments.
The following statistics indicate the hereditary possibility of covering the body with red lichen lesions. In the families of approximately 1% of patients, several generations suffered from lichen planus.
Can be prepared at home.
- For an ointment made from hops, burdock and calendula, take 1 tbsp. l. calendula flowers, 2 tbsp. burdock roots and hop cones. Boil in 200 g of water, cool, filter and mix with Vaseline in a proportion of 1 tbsp. l. decoction for 2 tbsp. l. Vaseline. The ointment is applied in a thin layer to the inflamed areas several times a day.
- To prepare the ointment, squeeze the juice from aloe leaves and mix it with 1 tbsp. l. incense and add a paste obtained from three cloves of garlic. Dip a tampon into the resulting mass and apply it to the area affected by lichen for 20 minutes.
- Honey ointment is considered an effective remedy against lichen. It is prepared from 15 g of propolis and 100 g of petroleum jelly. Frozen propolis is heated in a water bath for about a minute until completely dissolved, decanted and applied to skin affected by lichen ruber twice a day.
Essential and regular vegetable oils
Oils of various plants are used to soothe itching and relieve irritation of skin affected by lichen ruber:
- Sea buckthorn oil helps restore skin. A tampon soaked in it is applied to the mucous membrane or skin for 30-60 minutes several times a day.
- Calendula oil helps reduce swelling and remove redness.
- Olive oil soothes irritation and restores skin. It is used for exacerbation.
There are other ways to relieve inflammation when the skin is affected by lichen ruber. For example, beets. It is rubbed and tied to the affected area. As soon as the beets are dry, they are replaced with new ones.
Treatment of red lichen with clean hot water is effective. The affected area is doused with water 2 times a day for 3 minutes. Improvement can be seen after an average of 3 days. After 2 weeks, there may be no trace left of the lichen.
How to cure lichen planus?
The composition and tactics of treatment for lichen planus depend on the degree of spread of the lesion, on the cause and localization of the pathology, on information about the effectiveness of previously administered therapy. Thus, treatment of the disease involves the use of systemic and external medications, as well as phototherapy.
Among the most effective medicinal groups of drugs for the treatment of lichen planus, experts identify:
- antihistamines;
- multivitamin complexes;
- antifungal agents;
- systemic corticosteroids;
- systemic retinoids, etc.
Factors causing the disease
This disease is chronic and can be transmitted at the genetic level. However, among the reasons for its appearance or exacerbation, doctors name several factors:
- weakening of the immune system and increased infections;
- exposure to third-party chemicals (medicines, household and industrial preparations);
- the body’s own synthesis of dangerous components due to stressful situations;
- existing diseases (allergies, gastrointestinal disorders, damage to the mucous membrane, diabetes).
Accurate identification of the most likely causes of the disease subsequently determines the most effective method of treating lichen ruber.