Publications in the media
Lichen pilaris pityriasis versicolor is a non-contagious dermatosis of unknown etiology, characterized by follicular hyperkeratotic papules (follicular horny nodules) on the trunk and extensor surfaces of the extremities, peeling of the skin of the face and scalp, erythema and keratoderma of the palms and soles. Frequency. 0.03% of cases of all dermatoses.
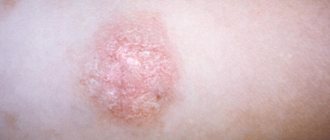
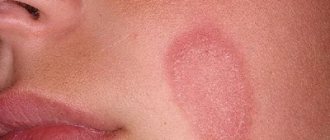
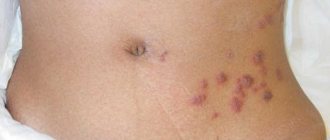
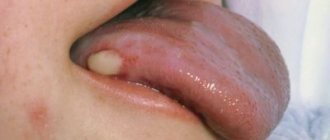
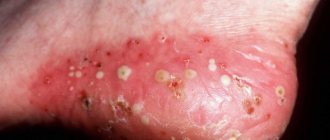
Clinical picture • Dermatosis most often begins with damage to the scalp in the form of erythematous-squamous lesions. Only after a few months or years do typical pointed follicular papules the size of a millet grain, yellowish-red in color, with a horny tip or covered with whitish scales appear, often located on reddened skin. In the center of the nodules you can always find twisted vellus hair. The nodules can merge into psoriasis-like infiltrated plaques, covered with abundant whitish, tightly packed scales. Increasing in number and size, these plaques merge into wide fields and can occupy the entire surface of the skin. • The rashes are symmetrical , localized on the extensor surfaces of the extremities in the area of the elbow and knee joints, on the face and neck. Typical are small pointed follicular horny papules on the dorsal surfaces of the 1st and 2nd phalanges of the fingers (Beignet cones). • When you touch the follicular rash, you get a feeling of a grater. • Damage to the palms and soles has the appearance of pronounced lamellar hyperkeratosis. Painful, deep cracks often appear. • Nails are often thickened , as if compressed from the sides, hyperkeratosis of the nail bed, clouding, longitudinal striations, and fragility of the nail plates are observed.
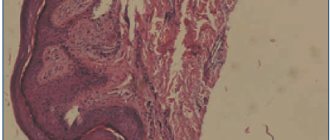
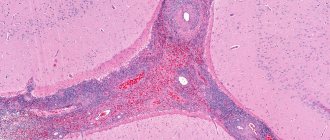
Research method . Skin biopsy - follicular hyperkeratosis with nests of parakeratosis over the papillae, uneven acanthosis, vacuolar degeneration of the basal layer; a slight infiltrate of the dermis, consisting of lymphocytes, neutrophils and a few mast and plasma cells, is located mainly around moderately dilated vessels and hair follicles.
Differential diagnosis • Psoriasis • Eczema • Secondary erythroderma • Mycosis fungoides.
TREATMENT Drug therapy • Complex therapy: vitamin E, retinol, cyanocobalamin, ultraviolet radiation, PUVA therapy, HA • HA ointments, creams. Course and prognosis • The course of the disease is chronic, with remissions and exacerbations • Often the disease begins acutely and in 2–3 weeks universal erythroderma with islands of unaffected skin can form • The prognosis is favorable. Prevention. Prevention and elimination of foci of focal infection; prevention and treatment of endocrine disorders and diseases of the nervous system. Synonyms • Devergie's disease • Lichen acuminata • Keratosis multiforme universalis • Devergie lichen acuminata
ICD-10 • L44.0 Pityriasis pilaris pityriasis rubra
To identify diseases and conditions that are contraindications to systemic therapy, before starting treatment and to identify adverse events associated with the therapy, patients are examined during treatment.
Isotretinoin
- When taken orally, regular monitoring of liver function and plasma lipid levels is necessary before treatment, 1 month after the start of therapy, and then every 3 months.
- For diabetes mellitus, obesity, alcoholism or lipid metabolism disorders, more frequent monitoring of laboratory parameters is recommended. If you have diabetes mellitus or are suspected of having it, it is necessary to strictly monitor plasma glucose levels.
- It is recommended to monitor liver function and liver enzyme levels in the blood before treatment, 1 month after the start of therapy, and then every 3 months or as indicated. If the level of liver transaminases exceeds the norm, it is necessary to reduce the dose of the drug or discontinue it.
- Fasting serum lipid levels should be determined before treatment, 1 month after initiation of therapy, and then every 3 months or as indicated. It is necessary to monitor the level of triglycerides in the blood serum, since a clinically significant increase in their level above 800 mg/dL or 9 mmol/L may be accompanied by the development of acute pancreatitis, possibly with a fatal outcome. If hyperlipidemia or symptoms of pancreatitis are persistent, isotretinoin should be discontinued.
- Due to the fact that while taking isotretinoin, a decrease in visual acuity, the development of keratitis, and dry conjunctiva are possible, patients with vision complaints should be referred to an ophthalmologist and discontinuation of the drug should be considered.
- Women must have a negative result from a reliable pregnancy test within 11 days before starting the drug. A pregnancy test is recommended monthly during treatment and 5 weeks after the end of treatment. It is recommended to use contraception.
Acitretin
- Liver function should be monitored before starting treatment with acitretin, every 1-2 weeks for the first month after starting treatment, and then every 3 months. If test results indicate pathology, monitoring should be carried out weekly. If liver function does not return to normal or worsens, the drug should be discontinued. In this case, it is recommended to continue monitoring liver function for at least 3 months.
- Fasting serum cholesterol and triglyceride levels should be monitored.
- In patients with diabetes mellitus, acitretin may worsen glucose tolerance, so in the early stages of treatment, blood glucose concentrations should be checked more often than usual.
- In children, it is necessary to monitor the parameters of bone growth and development.
- Due to the potential for impaired night vision, careful monitoring of visual impairment is necessary.
- Due to the high teratogenicity of acitretin, a negative pregnancy test result must be obtained 2 weeks before the start of treatment. During treatment, it is recommended to conduct additional pregnancy tests at least once a month. It is absolutely essential that every woman of childbearing potential use effective contraception without interruption for 4 weeks before starting treatment, during treatment, and for two years after completing treatment with acitretin.
Cyclosporine
Regular monitoring of plasma creatinine concentration is necessary - an increase may indicate a nephrotoxic effect and require a dose reduction: by 25% if the creatinine level increases by more than 30% from the original and by 50% if its level doubles. If dose reduction within 4 weeks does not lead to a decrease in creatinine levels, cyclosporine is discontinued. It is recommended to monitor blood pressure, blood levels of potassium, uric acid, bilirubin, transaminases, and lipid profile.
Azathioprine
- During treatment, women of childbearing age should use reliable methods of contraception.
- During the first 8 weeks of treatment, a clinical blood test should be performed weekly, including platelet determination (subsequently - 1-2 times a month), as well as periodic monitoring of the activity of serum liver transaminases, alkaline phosphatase and bilirubin levels.
- In the future, blood tests can be monitored less frequently, but a clinical blood test with platelet determination should be performed monthly or at least at intervals of no more than 3 months.
- Patients receiving azathioprine should be instructed to immediately report any infections, unexpected bruising, bleeding, or other signs of bone marrow suppression.
Methotrexate
- To reduce the likelihood of adverse events, treatment with methotrexate should be accompanied by oral folic acid 5 mg once a week 1-3 days after taking methotrexate.
- In order to timely identify side effects, it is necessary to monitor the condition of peripheral blood, for which a general blood test is performed once a week to determine the number of leukocytes and platelets. It is necessary to monitor the activity of liver transaminases, kidney function, and, if necessary, conduct an X-ray examination of the chest organs. Methotrexate therapy is stopped if the number of leukocytes in the blood is less than 1.5 x 109/L, the number of neutrophils is less than 0.2 x 109/L, and the number of platelets is less than 75 x 109/L. An increase in creatinine levels by 50% or more of the initial level requires repeated measurement of creatinine levels. An increase in bilirubin levels requires intensive detoxification therapy.
- If diarrhea and ulcerative stomatitis develop, methotrexate therapy should be interrupted. If signs of pulmonary toxicity (especially dry cough without sputum) appear, methotrexate treatment is recommended to be discontinued. Signs of bone marrow suppression, unusual bleeding or hemorrhage, black tarry stools, blood in the urine or stool, or pinpoint red spots on the skin require immediate consultation with a doctor.
- Men and women of childbearing age should use reliable methods of contraception during treatment with methotrexate and for at least 3 months after.
Biological therapy with infliximab, etanercept, adalimumab
Before starting biological therapy with infliximab, etanercept, adalimumab, the following studies are necessary:
- clinical blood test, including determination of leukocyte formula and platelet count;
- biochemical blood test, including a study of the level of creatinine, urea, bilirubin, determination of the activity of aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase and alkaline phosphatase;
- general urine analysis;
- X-ray of the chest organs in 2 projections, tuberculin tests, consultation with a phthisiatrician to exclude tuberculosis infection;
- examination for HIV infection, viral hepatitis B and C;
- pregnancy test.
Biological therapy is not recommended during pregnancy. Therefore, women of childbearing potential must obtain a negative pregnancy test before starting biological therapy. During biological therapy and for at least 6 months after its completion, women of childbearing age should use reliable methods of contraception.
During biological therapy, monitoring of adverse events and control of laboratory parameters are carried out:
- clinical assessment of the patient’s condition every 3-6 months;
- examination by a neurologist every 3-6 months to identify manifestations of neurological, including demyelinating, diseases;
- consultation with a cardiologist every 3-6 months to identify signs of cardiovascular failure;
- consultation with a phthisiatrician 2 times a year to exclude the development of tuberculosis;
- clinical blood test once every 3-6 months (for infliximab - before each intravenous administration);
- biochemical blood test (examination of the level of creatinine, urea, bilirubin, determination of the activity of aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase and alkaline phosphatase in the blood) once every 3-6 months (for infliximab - before each intravenous administration);
- general urine test once every 3-6 months (for infliximab - before each intravenous administration);
- blood test for hepatitis B, C and HIV infection - every 6 months;
- pregnancy test (for infliximab - before each intravenous administration), as well as if there is a possibility of pregnancy;
- X-ray of the chest organs 2 times a year;
- Ultrasound examination of the abdominal organs, pelvic organs, prostate gland according to indications.