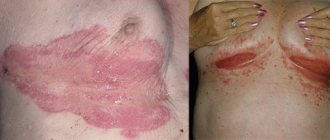
Psoriasis is a pathological immune-associated non-infectious dermatological disease. Nowadays, due to frequent stress, contact with harmful chemical compounds, difficulties in maintaining a daily routine and proper nutrition, this disease occurs in almost every twenty-fifth person. This disease is a chronic inflammatory process, which is characterized by periods of remission and relapse.
The main danger of this disease is that people susceptible to the disease ignore the first mild symptoms and do not seek help from specialists, preventing timely diagnosis in its early stages and the start of therapy. But psoriasis is diverse and can not only affect any part of the patient’s skin, but also nails and joints, subsequently leading to serious complications.
Palmoplantar psoriasis in the photo with description
Photo 1. Provoking factors for psoriasis: obesity.
Photo 2. Provoking factors for psoriasis: smoking and alcohol.
Photo 3. Psoriasis on the palms.
Photo 4. Psoriasis on the soles of the feet.
Photo 5. Symptoms of palmar psoriasis: peeling and the appearance of a red crust.
Photo 6. Symptoms of palmar psoriasis: peeling of the nail plates.
Causes of palmoplantar psoriasis
In most cases, the palmoplantar form appears due to congenital or acquired characteristics of the immune system or is transmitted genetically. A combination of both factors is possible.
Without provoking factors, the disease may not manifest itself at all. Here are the main reasons that can give rise to the appearance of a rash:
- past infections (viral and bacterial);
- the presence of inflammatory processes in the body;
- a course of medications (arsenic-based, beta-blockers, oral contraceptives, etc.);
- drugs, smoking and alcohol abuse;
- overweight, metabolic disorders;
- pathologies of the heart and blood vessels;
- disturbances in the functioning of the endocrine system;
- severe or prolonged stress.
With psoriasis, the immune system too actively produces protective substances, the aggression of which is directed at its own tissues and cells. As a rule, these are T lymphocytes. Their activity provokes inflammation and excessive cell division. This is what causes the symptoms. The risk group includes people from 30 to 50 years old.
There are different degrees of pathology, which is especially evident on the hands. Therefore, in the case of psoriasis on the palms, photos of the initial stage may differ.
Symptoms of palmoplantar psoriasis
Standard clinical manifestations:
- inflamed areas of pink or red color with clearly defined boundaries;
- severe peeling on top of the rash (clearly visible in the photo of psoriasis on the palms of the hands);
- painful cracks in areas with rashes (not always the case);
- pustules (rash with purulent contents, characteristic of some types of psoriasis);
- intense itching and burning (does not appear in all patients).
The disease has different forms. Therefore, some symptoms may vary.
If you discover suspicious symptoms, you should contact a dermatologist as soon as possible. You should not diagnose yourself by studying photos of palmoplantar psoriasis.
Types of palmoplantar psoriasis
There are two main types of palmoplantar psoriasis: ordinary and pustular (Barber type).
Ordinary psoriasis manifests itself as follows:
- In the area of the palms (often closer to the big toe and little finger) or soles (heel, lateral surface of the foot and area of the metatarsophalangeal joints), clearly defined, inflamed areas of reddish-yellow skin form. With severe exacerbation of the disease, the rash may occupy the entire palm or sole.
- in some cases, a large number of rough scales accumulate on the surface of the rash, forming deep and painful cracks
Pustular palmoplantar psoriasis (Barber psoriasis) is additionally characterized by the presence of pustules - intradermal accumulations of whitish-yellow pus. Purulent rashes can form both on top of plaques and on clean skin.
The pustular subtype develops quickly, is characterized by frequent relapses and is difficult to treat. Treatment for Barber psoriasis differs significantly from treatment for other subtypes.
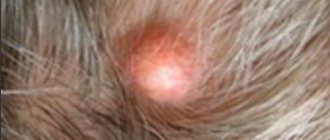
What is pustular psoriasis?
This type of psoriasis causes reddish, scaly, pus-filled bumps.
Anyone who has pus-filled bumps on most of their body needs immediate medical attention. When widespread, pustular psoriasis can be life-threatening.
Pus-filled bumps are called pustules. They can form on the skin, inside the mouth, or under the nail.
About 24 to 48 hours after the pus-filled bumps appear, they join together and burst.
When the pus dries, the area becomes red and glazed. It often feels damp and soft.
New pustules may form on the red glazed area. They too will join together and break apart.
Treatment methods for palmoplantar psoriasis
Palmar and plantar psoriasis requires complex treatment using systemic medications, external agents and physiotherapeutic techniques. Since it is impossible to completely get rid of psoriasis, therapy aims to relieve symptoms and prolong remission.
Systemic treatment involves the use of active anti-inflammatory drugs from the group of immunosuppressants (methotrexate, cyclosporine, etc.) or retinoids (acitretin).
For psoriasis on the palms and back of the hands, external treatment includes:
- glucocorticosteroids (hormonal creams and ointments) - this group of drugs most effectively copes with the inflammatory process in the skin and requires mandatory medical supervision during the treatment period.
- vitamin D and its analogues;
- non-hormonal external preparations - products containing naphthalan, salicylic acid, urea, tar are suitable for use both during exacerbation of the disease and during remission. This group of drugs rarely causes side effects and can be used independently by patients.
- emollient creams;
In addition to medications, physiotherapy is actively used. Typically it includes the following procedures:
- exposure to UV rays (natural or hardware);
- PUVA therapy;
- excimer laser treatment;
Treatment methods are selected by a dermatologist, guided by the severity of the disease and the characteristics of the patient’s body. The key difficulty in selecting drugs is their compatibility and contraindications. In addition, for therapy to be effective, medications must be rotated in terms of their level of anti-inflammatory activity. Therefore, you cannot self-medicate.
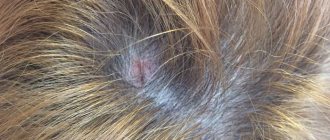
Continuous acrodermatitis Gallopo
This type of pustular psoriasis is rare. It causes pus-filled bumps on the fingertips, toes, or both. The disease can also develop under the nails.
Often, starting with one finger or toe, new pus-filled bumps may continue to appear. When this happens, new pustules may appear on more than just your fingers and toes. In rare cases, pus-filled bumps may slowly spread up the arms or legs.
Anyone who has pus-filled bumps on most of their skin needs immediate medical attention. When widespread, pustular psoriasis can be life-threatening.
The first choice for treating this type of pustular psoriasis often includes the following.
- Synthetic vitamin D combined with a strong corticosteroid: This combination is applied to the skin.
- PUVA: This is a type of light treatment that involves taking a medicine called psoralen before applying ultraviolet light to the affected skin.
Although the above describes what treatment may be used for each type of pustular psoriasis, your treatment plan may involve different medications. Your age, other medical conditions (if any) and overall health also play a key role in determining which treatment is best for you.
Literature used
Fitzpatrick J. “Pustular eruptions.” In: Fitzpatrick D.E. and Eling J.L. Secrets of dermatology. Hanley and Belfus, Inc., Philadelphia, 1996: 66-7.
Jeon K, Nakamura M, et al. “Generalized pustular psoriasis treated with apremilast in a patient with multiple comorbidities.” Representative for the JAAD case. 2017; 3(6): 495-7.
Robinson, Van Voorhis A.S. et al. “Treatment of pustular psoriasis: From the Medical Council of the National Psoriasis Foundation.” J Am Academy of Dermatology 2012; 67:279-88.
Source : https://www.aad.org/public/diseases/psoriasis/treatment/genitals/pustular
Posted by Dr. James Fitzpatrick
Complex therapy of palmoplantar psoriasis using Losterin cream
Dermatologists often prescribe classic cream or foot cream “Losterin” in complex therapy of the active stage of palmoplantar psoriasis, as well as to maintain the disease in remission. However, it can be freely purchased at a pharmacy or online. This is a local non-hormonal remedy. It does not contain aggressive and harmful components, as well as dyes and flavors. Therefore, “Losterin” is absolutely safe.
All products from the Losterin line have been tested in laboratories and confirmed their effectiveness. Doctors often recommend them due to the following advantages:
- ease of use (the drug can be used at home and while traveling without any problems).
- lack of addictive effect (the product can be used every day and for several courses);
- no side effects - the cream is well tolerated and does not cause allergic reactions
- complex effect on the symptoms of the disease - the drug reduces peeling on the surface of rashes, effectively copes with skin redness
- harmless composition;
Losterin gently fights inflammation and eliminates external symptoms.
Current trends in the treatment of palmoplantar psoriasis and acquired limited forms of keratoderma
S.V.
KLYUCHAREVA1, K.S. GUZEV2, V.I. NOZDRIN2 1 – Federal State Budgetary Educational Institution of Higher Education “North-Western State Medical University named after. I.I. Mechnikov", St. Petersburg, Russia, 195256; 2 – JSC “Retinoids”, Moscow, Russia, 111123.
Summary
Keratoderma is a group of diseases united by a common feature - increased keratinization due to an increase in the number of keratinocytes with a decrease in their normal desquamation. Excessive keratinization of the skin of the palms interferes with tactile sensitivity, which becomes a source of discomfort, and cracks lead to loss of performance.
Purpose of the study
— to study the possibilities of using the drug Uroderm for the treatment of patients with acquired limited forms of keratoderma.
Material and methods.
Two groups, equal in terms of the main characteristics, were formed: the 1st group included 37 patients (10 men and 27 women) with a diagnosis of palmoplantar psoriasis, the 2nd group included 39 women with a diagnosis of climacteric keratoderma. Participants in both groups used Uroderm ointment 2 times a day for 30 days.
Results.
A significant reduction in the area of lesions from the initial level was established in both groups (up to 44% in group 1 and up to 19% in group 2). There was a significant decrease in the values of the dermatological index of the symptom scale and the Visual Analogue Scale in group 1 - from 9.64±3.71 and 3.39±1.08 to 2.13±1.04 and 1.19±0, 83 points, respectively, in the 2nd group - from 10.31±3.45 and 3.67±0.97 to 1.01±0.62 and 0.57±0.57 points, respectively. The use of Uroderm ointment allowed, within a relatively short period, to achieve clinical recovery in participants of group 1 - 16 (43.2%) out of 37, or a significant improvement in the condition of representatives of group 2 - 31 (79.5%) out of 39.
Conclusion.
Uroderm ointment has a significant positive effect on the severity of the main objective clinical manifestations of limited forms of keratoderma. The drug has high safety and tolerability rates: representatives of both groups have no cases of complications; None of the study participants had any episodes of allergies or individual intolerances.
Keywords:
keratoderma, psoriasis, urea, Uroderm.
Keratoderma is a heterogeneous group of diseases characterized by diffuse or focal thickening of the stratum corneum of the epidermis of the palms and soles, in some patients in combination with keratoses of other localization, ectodermal dysplasia and inflammation [1–3]. Hereditary palmoplantar keratodermas have common histological features: acanthosis, hyperkeratosis, and sometimes focal parakeratosis. There are small perivascular infiltrates in the upper dermis. Hyperkeratosis and granulosis are observed in the vast majority of diffuse and limited forms of palmoplantar keratoderma [4].
For symptomatic purposes, keratolytics are used to treat keratoderma, one of which is urea. Urea in high concentration denatures and dissolves proteins, thereby providing a keratolytic effect, improves skin hydration and increases desquamation, and reduces skin itching. The advantage of urea is the absence of toxic and allergenic effects on the body. F. Julia et al obtained positive results when treating patients with dry flaky skin with urea preparations against the background of ongoing or previous dermatitis and patients with hand eczema. [5]. P. Oztas et al. [6]. It was found that in psoriasis, external treatment with urea reduces hyperproliferation of the epidermis and induces differentiation of keratinocytes.
Successful examples of the use of drugs with urea for the treatment of keratoderma have been noted, but since they are not yet numerous enough and are not widely used in practice, the search for optimal ways to solve this problem remains relevant. In this sense, the use of a domestic drug, which in its characteristics, content and properties is close to the idea of an ideal remedy for the successful treatment, control and prevention of acquired limited keratoderma (ALK), is promising.
Uroderm drug
- ointment for external use on an emulsion basis, which has a keratolytic effect, softens and moisturizes the skin, increasing its elasticity. The emulsion base of the ointment is easily absorbed by the stratum corneum of the skin, helping to soften the keratin. The concentration of urea in Uroderm ointment is 30% - this is higher than in any other product containing urea. Unlike various cosmetics with urea, Uroderm ointment is a medicinal product with a proven pharmacological effect, produced from pharmacopoeial quality components.
Purpose of this work
— substantiation of the feasibility of using the drug Uroderm as a pathogenetically justified, non-invasive drug for the treatment of patients with POC.
To achieve the goal of the study, standard criteria for assessing the effectiveness of therapy were used: the influence of the therapeutic agent used on the severity of the main clinical signs of the pathological process (criterion 1), the therapeutic effect (criterion 2), the incidence of side effects of therapy (criterion 3), the quality of life of patients in the comparison groups (criterion 4) and consumer properties of the drug (criterion 5).
The grounds for including patients in the clinical trial program were voluntary informed consent to participate in the study, confirmation of the diagnosis of palmoplantar psoriasis (PLP), climacteric keratoderma (KC), age from 21 to 69 years inclusive, the presence of indications and the absence of absolute contraindications to the use of the included into the external therapy program, the possibility of treating the patient on an outpatient basis and compliance with the agreed conditions of the trial, which imply strict adherence to medical recommendations and continued participation in the study until its completion.
Material and methods
The objects of observation were 76 outpatient patients (10 men and 66 women) aged from 21 to 69 years (average age 44.5±9.4 years), suffering from the most common nosological forms of non-hereditary POC, with no signs of a systemic process, with a duration diseases from 3 months to 20 years. The diagnosis of POC was initially made clinically. In order to verify it, the set of preliminary diagnostic measures, along with the collection of anamnesis and physical examination, included mandatory laboratory tests performed in accordance with the recommendations for the diagnosis of keratoderma [5].
Two groups of patients were formed: the 1st group included 37 patients (10 men and 27 women) with a diagnosis of palmoplantar psoriasis, the 2nd group included 39 women with a diagnosis of menopausal keratoderma. The criteria for analyzing the therapeutic effectiveness of the treatment method used in the groups were the following specific and nonspecific indicators.
Criterion 1.
Sign 1 - the dynamics of regression of objective symptoms of the disease, assessed by comparing the areas of affected areas before and after treatment. The current assessment of results is the registration and comparison of the areas of affected areas before and after treatment in absolute values. Presentation of results is a relative change in the areas of affected areas (decrease, increase), observed during treatment and expressed as a percentage of the initial level. Sign 2 is the dynamics of regression of objective symptoms of the disease, assessed by the results of changes in the dermatological index of the symptom scale at each control point of observation. Sign 3 - dynamics of the subjective indicator (index) of the severity of itching, calculated on a 10-point Visual Analogue Scale (VAS) - the most constant sign characteristic of both diseases under consideration on the 10th, 20th and 30th days of observation.
Criterion 2.
Global assessment of the therapeutic effect (adaptation option in relation to the conditions of this study). Comparative analysis of the effectiveness of treatment of POC using the drug Uroderm. Evaluation of results in groups according to the number of patients who achieved clinical recovery (excellent treatment result - a decrease in the severity of a clinical sign as a percentage of 75% or higher from the initial level), significant improvement (a good treatment result - a decrease in the severity of a clinical sign as a percentage of 50 to 75% of the initial level), moderate improvement (satisfactory treatment result - reduction in the severity of the clinical sign as a percentage from 25 to 50% of the initial level), no effect (unsatisfactory treatment result - reduction in the severity of the clinical sign as a percentage of less than 25% of the initial level) level). Presentation of results - assessment of the severity of the studied phenomena before and after treatment (in points). To ensure the reliability of the results obtained during statistical processing of the data, a ranking method was used: an excellent result corresponded to 3 points, a good result corresponded to 2 points, a satisfactory result corresponded to 1 point, and an unsatisfactory result corresponded to 0 points. The assessment control point is the 30th day of observation.
Criterion 3.
Frequency of development of undesirable effects of treatment, comparative assessment of the safety and tolerability of the drug Uroderm in the treatment of POC. Methods for obtaining information: clinical observation data (objective method), questionnaires (subjective method).
Criterion 4.
Changes in the assessment of quality of life based on the dynamics of the Dermatology Life Quality Index. Assessment of psychosomatic status in patients with POC before and after treatment. Analysis of the strength of influence of the only external therapy used in the experiment on the level and quality of physical, mental, social and role-related or functionally associated lifestyle on patients with diseases of various natures. Method: survey. The method is to independently fill out a universal form of a standard questionnaire, which is a Russified version of the Dermatology Life Quality Index or the dermatological quality of life index (DIQL). Control points: 1st and 30th day of observation.
Criterion 5.
Assessing the results and quality of treatment, analyzing consumer properties, studying the reaction and attitude of patients to the use of a new external therapy. Method: survey. The method is to independently fill out a simple questionnaire that allows you to assess patient compliance and further prospects for using the drug in patients with various nosological forms of POC.
Statistical processing was carried out using generally accepted methods of parametric and nonparametric statistics (Excel and Statistica 6.0 programs). To compare qualitative, quantitative and semi-quantitative characteristics, χ2 and Wilcoxon-Mann-Whitney tests were used. Data were presented in the form of absolute or relative (%) values, quantitative - in the form of X±x, where X is the arithmetic mean, x is the error of the average value. The difference in values was considered significant at p
Results and discussion
There were no significant differences in the severity of the assessed clinical manifestations of the disease, as well as significant differences in the assessment of the quality of life at the pre-treatment stage in representatives of both groups: the initial indicators in the comparison groups were statistically indistinguishable (p
Criterion 1.
The strength of the influence of the therapy used on the severity of the main clinical signs of the pathological process (Table 2).
Despite the fact that as a result of treatment, patients in both groups showed positive dynamics, the strength of the effect of therapy on the assessed clinical sign of the pathological process turned out to be different.
Thus, in patients of group 1, the use of the declared drug led to a reduction in the lesion area by 56%, which by the end of the study amounted to 44% of the initial level, which can only be interpreted as a clinically significant positive result (p>0.05). At the same time, patients in group 2 showed a more pronounced reduction in the value of the studied parameter - up to 19% of the initial level (p Table 1.
Distribution of patients by group
| Index | Group | |||||
| 1st (n=37) | 2nd (n=39) | Total | ||||
| abs. | % | abs. | % | abs. | % | |
| Number of participants | 37 | 39 | 76 | 100 | ||
| men | 10 | 27,0 | — | — | 10 | 13,2 |
| women | 27 | 73,0 | 39 | 100 | 66 | 86,8 |
| Area of lesions, cm2 | 32,5±15,7 | 38,9±17,4 | ||||
| DISHS | 9,64±3,71 | 10,31±3,5 | ||||
| VAS | 3,39±1,08 | 3,67±0,97 | ||||
| Dermatology Life Quality Index | 12,2±1,3 | 12,0±1,0 | ||||
Table 2.
Change in the area of lesions during treatment
| Group | Change in horizontal area of lesions, cm2 | |||||||
| 1 | 2 | 4 | ||||||
| abs. | % | abs. | % | abs. | % | abs. | % | |
| 1st (n=37, N=107) | 32,5±15,7 | 100 | 27,6±12,3 | 85 | 19,6±7,8 | 60 | 14,3±6,5 | 44 |
| 2nd (n=39, N=120) | 38,9±17,4 | 100 | 26,4±13,9 | 68 | 10,7±5,8* | 28 | 7,4±3,4* | 19 |
Note.
Here and in Tables 3, 6: * - within the 95% CI, intragroup differences in indicators relative to the initial level are statistically significant (p
Table 3.
Dynamics of objective and subjective symptoms of disease severity during treatment
| Indicator/index | Group | |||||||
| 1st (n=37) | 2nd (n=39) | |||||||
| observation control points (points) | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| DISHS | 9,64±3,71 | 6,28±2,21 | 4,89±1,28*# | 2,13±1,04* | 10,31±3,45 | 4,10±1,02* | 2,53±0,96*# | 1,01±0,62* |
| VAS | 3,39±1,08 | 2,45±0,71 | 1,50±0,67* | 1,19±0,83* | 3,67±0,97 | 1,75±0,66* | 1,09±0,51* | 0,57±0,57* |
Note.
Here and in the table. 6. # - within the 95% CI, intergroup differences in indicators are statistically significant (p
In addition, in group 2, the intensity of the decrease in the value of the studied parameter was more pronounced than in group 1. Thus, a statistically significant reduction in the affected area was observed already by the 20th day of the study.
Preliminary conclusions about the effectiveness of the use of the drug Uroderm in the treatment of POC were also confirmed by analysis of changes in the severity of other clinical symptoms of the disease (Table 3).
During treatment, the average values of DISH and VAS in group 2 decreased statistically significantly from 10.31±3.45 and 3.67±0.97 to 1.01±0.62 and 0.57±0.57 points, respectively (p
Criterion 2.
Global assessment of therapeutic effect (Table 4).
Comparative analysis of the data given in table.
4 demonstrates the differences in treatment results in patients of the 1st (Fig. 1, a, b) and 2nd (Fig. 2, a, b) groups. The advantages of using the drug in group 2 are quite obvious. This is evidenced by the fact that the total proportion of representatives of the 2nd group who achieved clinical recovery - 8 (20.5%) out of 39 and significant improvement - 23 (59%) out of 39 was 79.5%, while as in group 1, the total proportion of study participants who achieved a similar result was almost 2 times less and amounted to only 43.2% (5.4 and 37.8%, respectively). Table 4.
Comparative analysis of the effectiveness of using Uroderm ointment as a monotherapy in patients with palmoplantar psoriasis and climacteric keratoderma
| Result | Group | |||
| 1st (n=37) | 2nd (n=39) | |||
| abs. | % | abs. | % | |
| Excellent (3 points) | 2 | 5,4 | 8 | 20,5 |
| Good (2 points) | 14 | 37,8 | 23 | 59,0 |
| Satisfactory (1 point) | 18 | 48,7 | 7 | 17,9 |
| Unsatisfactory (0 points) | 3 | 8,1 | 1 | 2,6 |
| Average treatment effectiveness score | 1,41 | 1,97 | ||
Criterion 3.
Frequency of development of unwanted side effects of therapy (Table 5).
The data given in table. 5 demonstrate differences in the incidence of undesirable treatment effects in groups when using the studied external therapy. Analysis of the presented results indicates both a greater number of possible complications that arose in representatives of the 1st group, and a higher frequency of cases of their registration than in the 2nd group.
Fig 1.
Patient S., 52 years old, with a limited form of climacteric keratoderma. a — clinical picture of severe hyperkeratosis of the right foot before treatment; b — significant reduction in hyperkeratosis in the area of the right foot after treatment with Uroderm ointment (after 2 weeks of daily application).
Fig 2.
Patient O., 48 years old, with palmoplantar psoriasis.
a - pronounced hyperkeratosis, cracks; b - clinical improvement: epithelization of cracks, reduction of hyperkeratosis, restoration of the structure of the epidermis. Table 5.
Comparative analysis of the safety and tolerability of using Uroderm ointment in patients with palmoplantar psoriasis and climacteric keratoderma
| Symptom | Group | |||
| 1st (n=37) | 2nd (n=39) | |||
| abs. | % | abs. | % | |
| Paresthesia (itching, pain, tingling, feeling of tightness) | 3 | 8,1 | 2 | 5,1 |
| Edema, hyperemia | 3 | 8,1 | 2 | 5,1 |
| Vesicular eruptions, oozing | 2 | 5,4 | — | — |
| Depigmentation, hyperpigmentation | 4 | 10,8 | — | — |
| Peeling | 1 | 2,7 | 1 | 2,6 |
| Telangiectasia | 1 | 2,7 | — | |
| Allergic reactions, individual intolerance | — | — | ||
| Total | 14 | 37,8 | 5 | 12,8 |
Table 6.
Analysis of the strength of influence of the used therapy method (drug) on the quality of life of patients by group
| Chapter | Questions | Group | |||
| 1st (n=37) | 2nd (n=39) | ||||
| before | after | before | after | ||
| 1. Symptoms and sensations | 1 and 2 | 2,1±1,8 | 1,1±0,7 | 1,9±1,2 | 0,6±0,2 |
| 2. Daily activities | 3 and 4 | 1,9±1,7 | 1,0±0,4 | 1,7±1,4 | 0,7±0,3 |
| 3. Rest (leisure) | 5 and 6 | 1,6±1,2 | 0,8±0,4 | 1,5±1,3 | 0,5±0,3 |
| 4. Work and study | 7 | 1,3±0,9 | 0.7±0,5 | 1,4±0,6 | 0,4±0,2* |
| 5. Interpersonal relationships | 8 and 9 | 3,2±1,4 | 1,2±0.7 | 3,2±1,1 | 0,8±0,3* |
| 6. Treatment | 10 | 2,1±0,8 | 2,1±1,1 | 2,3±0,7 | 1,2±0,5 |
| Sum of points | 12,2±1,3 | 6,9±0,6*# | 12,0±1,0 | 3,7±0,3*# | |
Table 7.
Differences in assessing the consumer qualities of Uroderm ointment by group, abs. (%)
| Subjective comments | Comparison group | |||
| 1st (n=37) | 2nd (n=39) | |||
| Yes | No | Yes | No | |
| Presence of side effects | 14 (37,8) | 23 (62,2) | 5 (12,8) | 34 (87,2) |
| Ease of use | 19 (51,4) | 18 (48,6) | 36 (92,3) | 3 (7,7) |
| Availability of additional wishes | 16 (43,2) | 21 (56,8) | 7 (17,9) | 32 (82,1) |
| Satisfaction with the results of therapy | 22 (59,5) | 15 (40,5) | 34 (87,2) | 5 (12,8) |
| Willingness to continue therapy | 26 (70,3) | 11 (29,7) | 36 (92,3) | 3 (7,7) |
Criterion 4.
DIQL assessment (Table 6).
A detailed analysis of the DIQL (see Table 6), carried out in the main sections, showed the presence of clinically significant differences in the assessment of the quality of life before and after treatment by the absolute majority of patients in both groups. The exception was the differences in assessing the degree of influence of the treatment method on this indicator. Thus, if the participants of the 1st group, taking into account previous experience of treatment, did not change their attitude towards it even after these clinical studies (the average score before and after treatment was 2.1 ± 0.8 and 2.1 ± 1.1, respectively), then in patients of group 2 we can state clear positive changes in assessing the effect of therapy on their quality of life (2.3±0.7 and 1.2±0.5 points before and after treatment, respectively). In patients of both groups, a statistically significant decrease in the total indicator (index) of quality of life was registered after therapy: in group 1 - from 12.2±1.3 to 6.9±0.6 points (p
Criterion 5.
Assessment of consumer properties of Uroderm ointment (Table 7).
In a comparative analysis of the consumer properties of Uroderm ointment, the following data were obtained: participants in group 2 were much less likely (in 12.8% of cases) than representatives of group 1 (37.8%) to focus on the presence of side effects of treatment. Significantly less frequently (7.7% versus 48.6%, respectively) made complaints about the ease of use of the drug. Objectively, they expressed additional wishes during treatment less often (17.9% versus 43.2%). Significantly more often (87.2 and 59.5%, respectively) positively assessed the results of therapy and expressed readiness to continue it to the same extent (92.3% versus 70.3%, respectively).
conclusions
The drug Uroderm has a positive effect on the severity of the main objective clinical manifestations of diseases. The drug Uroderm really has high levels of safety and tolerability. The drug Uroderm has a pronounced positive effect on the quality of life of patients with POC who use it as monotherapy for their disease. The drug Uroderm has high consumer characteristics.
The authors declare no conflict of interest.
Literature
- Mordovtsev V.N., Mordovtseva V.V., Mordovtseva V.V. Hereditary diseases and malformations of the skin. M. 2004.
- Isaeva D.R., Haldin A.A. On the issue of differential diagnosis of hyperkeratotic dermatoses of the palmoplantar localization. Clinical dermatology and venereology. 2016;6:120-126.
- Kruglova L.S., Zhukova O.V., Fineshina E.I. Pathogenetic aspects underlying palmoplantar keratoderma. Modern methods of therapy. Clinical dermatology and venereology. 2015;14(2):17-23.
- Paltsev M.A., Potekaev N.N. Clinical and morphological diagnosis. M. 2004.
- Julia F, Phan A, Balme B, Thomas L. Severe palmoplantar keratoderma. Arch Dermatol. 2010;146:667-672.
- Oztas P, Alli N, Polat M, et al. Punctate palmoplantar keratoderma (Brauer–Buschke–Fischer syndrome. Am J Clin Dermatol. 2007;8:113-116.
Prevention of palmoplantar psoriasis
Many of the signs of psoriasis on the palms are also common on the feet and soles. Therefore, therapy and prevention are similar.
Basic preventive measures:
- Take daily baths with the addition of natural esters, sea salt or mild baby soap without any additives. Instead of baths, you can do 20-minute baths for your feet and hands.
- Use of moisturizers. Especially after a shower or bath. The product is applied once a day. In advanced cases - twice a day.
- Use soft towels. In this case, the skin is not rubbed, but “blotted” by carefully applying a towel to it.
- Walks in the open air. It is important for people with psoriasis to get enough sunlight. However, moderation is important here. Too much sun will only make the situation worse. Therefore, it is worth consulting a doctor about this issue.
- Rejection of bad habits. Cigarettes, alcohol and lack of daily routine.
- Protection against infections. It is important to monitor your immunity and not have contact with sick people.
- No stress. Or working on changing the perception of negative situations.
These rules must be followed throughout your life. Then the manifestations of the disease will be minimal, and the periods of remission will be longer.
Cost of treatment
Initial appointment with a dermatologist (assessment of patient complaints, history taking, examination, preliminary diagnosis, consultation)
Primary appointment – visiting a doctor of a specific specialty for the first time. Make an appointment
1170 ₽ 1500 ₽
Repeated appointment with a dermatologist
Make an appointment
620 ₽ 800 ₽
Treatment of mild psoriasis for 7 days (7 Dermalight procedures or 7 injections with our drugs + ointment from our components)
Make an appointment
4000 ₽
Treatment of moderate psoriasis for 7 days (3-6 droppers; 4 injections with our drugs; ointment from our components; 4 Dermalight procedures)
Make an appointment
5500 ₽
Treatment of severe psoriasis for 7 days (6-7 droppers or 12 injections with our drugs; ointment from our components; 4-6 Dermalight procedures)
Make an appointment
8000 ₽
Note: Most often, treatment of any grade requires a minimum of 4 weeks of treatment.