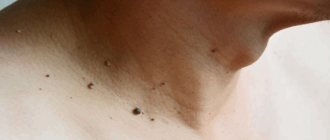
Human papillomavirus is one of the most common, along with herpes, chronic infections. Therefore, HPV testing is in great demand in the diagnosis of many diseases. Tests for all types of human papillomavirus are carried out in our clinic daily.
The main types of human papillomas:
- Skin papillomas: Ordinary thread-like, on a thin stalk. Most often located in the armpits, neck, eyelids, mammary glands and groin
- Flat papillomas are the color of the surrounding skin and rise slightly above it. They are mainly found on the fingers of the limbs and hands.
- Warts are papillomatous formations with keratinization on the surface. They can be everywhere, but more often on the limbs. They especially cause problems when they are located on the soles of the feet and around the nails.
All these types of papillomas develop in the presence of various types of human papillomavirus, detected using special laboratory diagnostics, which will be discussed.
Causes of the disease
A virus is an infection, which means that in order for it to appear in the body, infection must occur.
HPV penetrates the skin and mucous membranes of a healthy person upon their contact with the papillomas of a patient or with visually unchanged skin and mucous membranes of an infected person, provided that there is a large amount of virus at the site of contact and there is damage to the integrity of the epithelium (abrasions, scratches, wounds, erosions, sores). HPV infection is especially common in the presence of herpes simplex rashes, through which the human papillomavirus penetrates the skin and blood.
What tests should I take for HPV?
In our clinic we perform the following tests for human papillomavirus:
- PCR analysis for HPV types 6 and 11 - a scraping is taken from rashes or mucous membranes of interest to the doctor. The material is placed in a test tube and examined within 1–2 days. Depending on how the analysis is performed, the answer may be:
- Positive - when a virus is detected, indicating the specific type
- If a quantitative method was used, then indicate how many viral particles were detected
Negative - in the absence of HPV in the test material
HPV viruses types 6 and 11 belong to the group of low oncogenic (carcinogenic) risk and, basically, only cause the growth of genital warts or genital warts, which rarely develop into cancer.
The price of analysis for HPV types 6 and 11 is from 300 rubles. for qualitative analysis.
The result of the diagnosis can be either the detection of a certain type of virus or its quantity.
Human papillomaviruses types 16 and 18 are among the most malignant in terms of oncogenic risk and more often than others lead to the development of cancer of the cervix, skin and mucous membranes. Therefore, they are allocated to a separate diagnostic group.
The cost of testing for HPV types 16 and 18 is from 300 rubles. for a qualitative diagnostic option, 800 rubles for a quantitative one.
The analysis period is from 1 day.
- Qualitative without determining the type - the analysis will simply show whether there are viruses of these types in the scraping of cells without detailing
Qualitative with type determination (with genotyping) - the result will contain an indication of the presence of a specific type of HPV
The cost of analysis is from 900 rubles. for a quality option.
How to identify the HPV virus
In order to prevent fatal developments, it is necessary to be tested for HPV from time to time.
If suspicious foci are identified, no matter where - on the cervix in women or on the head of the penis in men, it is necessary to identify the type of pathogen (there may be several of them) and determine the number of virions.
Quantitative analysis of HPV also helps to make a forecast for malignancy (degeneration of the formation into cancer) - the more copies of the oncogenic HPV type in the sample, the higher the risk.
In modern dermatovenerology, it may be recommended to undergo several types of analyzes and tests:
- Cytology. This microscopy of cells from a suspicious lesion can find signs of cancer. Not suitable for identifying the type of viruses or early diagnosis at the stage of precancerous phenomena.
- Colposcopy with treatment of the lesion with acetic acid and Lugol's solution. Allows you to accurately determine the boundaries of the lesion.
- PCR analysis for HPV. An excellent molecular genetic method, which we will look at a little below.
- Serological tests. They are not very informative for clinical practice; they are more often used to conduct epidemiological studies on HPV.
Throughout the civilized world, a key role in the diagnosis of human papillomavirus infection is given to molecular genetic methods - PCR, Digene test.
When is the material collected?
The method of collecting material for diagnosis and the material itself in general depend on the manifestations of human papillomavirus infection.
If there are elements on the skin and visible mucous membrane, then using a universal urogenital probe, a dermatovenerologist or gynecologist scrapes cells from the surface of the papilloma. The resulting cells are placed in a tube for delivery to the laboratory and PCR analysis for HPV. The manipulation is absolutely painless and takes about 30 seconds.
When diagnosing papillomavirus infection in the urethra, vagina, cervical canal, especially in the presence of cervical erosion, scraping is carried out from these organs, preferably from the erosion itself.
If papillomas are present on the mucous membrane of the oral cavity or rectum, then material is taken from these areas with a probe.
If it is necessary to diagnose diseases of the prostate gland or bladder, urine and prostate secretions can be taken for HPV.
How is an HPV test done by scraping?
This diagnosis depends little on previous training. Although it would be nice if you exclude the effect of antiseptics on the skin and mucous membranes from which the doctor will scrape cells.
At an appointment with a dermatovenerologist, venereologist, urologist or gynecologist, epithelial cells are collected using a special probe from the area of interest:
- Urethra
- The head of the penis, especially rashes, erosions, ulcers on it
- Skin of the penis with rashes
- Skin of the anal area, rectal mucosa
- Labia majora and labia minora
- Vaginal mucosa
- Cervical canal
- Vaginal part of the cervix
- Mucous membranes of the cheeks, tongue, lips, gums
- Mucous membrane of the oropharynx, nasopharynx, larynx.
All manipulations are absolutely painless and low-traumatic.
Only the doctor collects the material! In our clinics, we do not even trust nursing staff with this procedure, because for the quality of the analysis, the scraping itself is even more important than further research.
When examining for human papillomaviruses, you need to take into account, among other things, the following points:
- HPV causes not only the growth of papillomas, condylomas, the development of erosions and cervical cancer. It is associated with a lot of other diseases and clinical manifestations on the skin and mucous membranes.
- Therefore, it is worth listening to a venereologist, urologist or gynecologist, who, for a certain clinical picture in a patient, prescribes tests for HPV of one type or another, or a complex test for HPV (human papillomavirus).
- The same person may have several types of HPV, and not all of these types will necessarily manifest themselves simultaneously clinically and be detected in the analysis.
- Therefore, after treatment, it is necessary to undergo a detailed test for HPV, and not be limited to one type, which was determined during the initial diagnosis.
- Sexual partners of patients infected with human papillomavirus are also tested for all types of HPV.
Only your doctor will determine what types of HPV tests you need to take. This depends on the clinical manifestations of the infection, the type of papillomas, the location of the elements and the results of the examination of the partner, if any.
| (20.6 KB in archive, MS Word format) | “Laboratory diagnostics” No. 1(9) 2013 |
| WHEN PUBLISHING MATERIALS, A LINK TO THE SOURCE IS MANDATORY!!! | |
| SUBSCRIBE TO THE MAGAZINE | |
Enzyme immunoassay in the complex diagnosis of herpesvirus infections
T.I. Dolgikh – Head of the Central Research Laboratory, Head
Academic Center for Laboratory Diagnostics Omsk State Medical Academy, Doctor of Medical Sciences, Professor.
State budgetary educational institution of higher professional education
“Omsk State Medical Academy” of the Ministry of Health of the Russian Federation
Against the backdrop of an increase in immunodeficiency, allergic and autoimmune diseases, herpesvirus infections are observed, which can form severe pathologies of various organs and systems, lead to disability and cause death. Among this group of infections, the most significant are herpes (HSV infection) and cytomegalovirus infections (CMV infection), as well as infection caused by the Epstein-Barr virus (EBV infection). Given the widespread prevalence of these pathogens and a similar clinical picture, the question of the optimal diagnostic algorithm becomes relevant.
To select patient management tactics, it is fundamentally important to establish the phase of the infectious process and assess the adequacy of the host’s immune response to pathogens. The tactics of doctors to identify opportunistic infections often have a one-sided approach and are often based on the determination of specific antibodies of the IgG and IgM classes (in some laboratories only a qualitative assessment of total antibodies is given), which often “drives” the doctor into a “serological dead end” and leads to hypo- or overdiagnosis.
Despite the relatively high cost of the tests, the use of a set of laboratory tests is quite justified, since the etiological interpretation of the diagnosis fundamentally influences the patient’s management tactics, determines the need for etiotropic and immunomodulatory therapy, and in some cases allows one to predict the outcome. Rational use of test systems based on the proposed principles at individual stages of patient examination allows one to avoid unnecessary multiple studies.
Along with polymerase chain reaction (PCR), aimed at detecting genetic
When identifying potential pathogen material, enzyme-linked immunosorbent assay (ELISA) is still widely used. It is aimed at identifying antibodies of various classes (IgM, IgA, Ig) in blood serum, plasma, and cerebrospinal fluid. Determination of total antibodies in the diagnosis of herpesvirus infections has low diagnostic value, provides only preliminary information about the presence of infection and requires further laboratory clarification.
To confirm the diagnosis and monitoring, immunoblot (Western-blot, Line-blot) is increasingly used, aimed at identifying antibodies to individual antigens of pathogens of herpes viral and other infections.
Immunoglobulin classes
IgM are the “earliest” antibodies, since they are formed in the early stages of the infectious process. They are able to agglutinate bacteria, neutralize viruses, activate complement and play an important role in eliminating pathogens from the bloodstream. They do not penetrate the placenta, are synthesized in the fetus and belong to the newborn’s own antibodies. Their presence indicates infection
(including intrauterine), indicates an active process. IgM antibody levels may increase during reactivation, reinfection, or superinfection.
IgG - play a fundamental role in humoral immunity in infectious diseases, causing the death of the pathogen with the participation of complement and opsonizing phagocytic cells. The IgG2 subclass is of greatest importance in anti-infective protection. IgG crosses the placenta and forms anti-infective immunity in newborns.
In recent years, it has become possible to determine the so-called “early” specific IgG, which have low avidity and indicate a primary infection (this test is used in the diagnosis of CMV infection, HSV infection). High-avidity antibodies are an indicator of long-standing infection and previous infection. The avidity of antibodies in sera is assessed by the avidity index (AI), which is expressed in %. Detection of DNA or antigens (“early proteins”) of the pathogen against the background of high-avidity antibodies indicates a persistent infection and indicates the activity of the infectious process.
IgA exists in two forms: secretory and serum. It does not pass through the placenta. Secretory IgA is found in milk, colostrum, saliva, lacrimal, bronchial and gastrointestinal secretions, bile, and urine. This is the main type of immunoglobulins involved in local immunity. The synthesis of serum IgA begins at the end of the first month of the disease
and continues as long as the antigen is available to immunocompetent cells. Its detection indicates an acute or subacute process, reactivation and superinfection. The test is indicative in the diagnosis of congenital forms of infections, since IgA does not pass through the placenta, but is produced in the child’s body in response to exposure to an infectious agent. It is advisable to use it to determine the activity of the infectious process in CMV, HSV and EBV infections.
Reasons for false-positive ELISA results
As with other reactions, a false-positive result is possible with ELISA. The causes of false-positive reactions should be divided into the following groups:
- errors at the preanalytical stage associated with violation of the rules for taking biomaterial (it is recommended to collect blood in plastic tubes intended for serum, preferably with a blood clot), hemolysis, bacterial contamination of blood or serum (occurs in case of violation of the temperature regime during storage or transportation ) test tubes with biomaterial;
— errors at the analytical stage in case of disruption of the technological process, as well as those depending on the quality of test systems and personnel qualifications;
— errors at the post-analytical stage (depending on the responsibility and qualifications of the personnel).
Particular attention should be paid to the causes of false-positive results that do not depend on laboratory staff. The most often incorrect one occurs when determining specific antibodies of the IgM class and may be due to the presence of rheumatoid factor, hyperproduction of IgM during pregnancy, cross-reaction with antigens of other pathogens, autoimmune process, metabolic disorders, lipidemia, etc. In this case, the use of a number of laboratory techniques allows one to obtain reliable analysis result. These primarily include: removal of rheumatoid factor from serum, use of highly specific foreign-made test systems at the first stage of production or as confirmatory tests, re-examination of serum, use of IgM immunoblot (or line blot) as a confirmatory test, aimed at identifying antibodies of the IgM class specifically to individual antigens of the pathogen. Along with this, it should be remembered that IgM is an indicator of an acute and active infectious process. Detection of pathogen DNA or its antigens by direct methods or the appearance of antibodies of other classes as a result of switching antibody synthesis over time allows the diagnosis to be verified.
When detecting IgG antibodies, a false-positive result can be obtained, which is primarily associated with cross-reaction of antigens (for example, between CMV and HSV) and directly depends on the specificity of the test systems. To clarify the laboratory diagnosis and exclude a false-positive result, an immunoblot (Westernblot-IgG, Line-blot), designed to detect IgG to individual highly specific pathogen proteins, should be used. Line-blot is distinguished by the fact that specific proteins with high diagnostic information are applied to or enhanced by them on a nitrocellulose membrane.
The immunoblot method (Westernblot, Line-blot, recom-Line) is a new generation of reference methods, highly specific and highly sensitive, confirming (or excluding) the diagnosis if infection is suspected if positive or questionable (uncertain) results are obtained when examining blood serum or cerebrospinal fluid in ELISA. Allows the determination of antibodies to individual pathogen antigens. It is an individual evaluation template (nitrocellulose membrane) with individual proteins (antigens) applied. Has the ability to automate research. To evaluate the signal, the position and intensity of the coloring of the bands are taken into account.
In case of CMV, HSV and EBV infections, to establish the activity of the infectious process, one should: 1) take into account the peculiarities of the formation of the immune response of patients of different age groups; 2) take into account diagnostically significant dynamics (an increase in indicators by 2-3 orders of magnitude), if the time factor allows this and prompt diagnosis is not required (!); 3) use a set of laboratory tests depending on the purpose and objectives of the study, including (especially if rapid diagnosis is necessary, identification of IgA antibodies, low-avidity IgG (in case of initial detection) and IgG in the cerebrospinal fluid (in case of damage to the central nervous system); 4) exclude co-infection herpes viruses; 4) in pregnant women, determine the presence of risk factors for infection of the fetus.
Features of laboratory diagnosis of intrauterine infection (IUI)
If a child is suspected of having IUI, tests are most often carried out for the presence of markers of herpesvirus infections. Examination of the mother during pregnancy in most cases allows us to exclude those infections for which negative results were obtained and conduct a targeted examination of the child. However, we must remember about the possibility of infection of a woman in late pregnancy, which in conditions of chronic placental insufficiency and secondary immunodeficiency (including HIV infection) leads to infection of the fetus or newborn.
If the mother has not been previously examined, then for a quick and more reliable diagnosis of IUI, it is recommended to conduct a parallel blood test of the mother and child. In this case, various situations are possible that cause difficulties for doctors in interpreting the results.
It is in the diagnosis of neonatal pathology that additional tests (determination of low-avidity antibodies, IgA, antigens and (or) pathogen DNA) have the greatest diagnostic significance. Detection of IgG alone is uninformative due to the circulation of maternal antibodies received transplacentally by the child (“immune contribution of a pregnant woman”). It is recommended to determine IgM, IgA, DNA or pathogen antigens, evaluate clinical data, and compare the results of general clinical and functional examinations.
It should be remembered that when examining newborns for IUI, a false negative result of a serological test may be obtained as a result of the influence of a high concentration of maternal antibodies of the IgG class (mask the presence of IgM in the child) or immunological tolerance, therefore it is preferable to use direct diagnostic methods aimed at detecting pathogen antigens (RIF ) and its nucleic acid (PCR) in the blood or cerebrospinal fluid (subject to spinal puncture for medical reasons). Immunological tolerance refers to the body's inability to mount an immune response to a specific antigen. The timing of its formation varies from several hours to several days; the duration depends on the persistence of the antigen in the body and the rate of formation of immunocompetent cells from their precursors; Induction of tolerance is facilitated by nonspecific immunosuppression (including under the influence of drugs). Tolerance can occur due to antigenic overload; it is not permanent; its duration can be increased by periodic exposure to antigen. Exit from the state of tolerance can be spontaneous or induced.
The detection of specific antibodies of the IgM and/or IgA class in young children clearly indicates that the child is infected (IgM and IgA are not transmitted through the placenta and are their own antibodies, produced in the presence of pathogen antigens). Our data indicate the difficulty of diagnosing the congenital form of opportunistic infections and the rare detection of IgM in newborns and young children. If neurological symptoms predominate, the level of specific IgG antibodies in the blood serum may not be detected or may be low.
In this case, the greatest diagnostic value is the study of cerebrospinal fluid for the presence of specific antibodies or antigens of the pathogen.
The reasons for a false-negative serological test may be: 1) immunological tolerance (more often); 2) the influence of a high concentration of maternal antibodies of the IgG class (they mask the presence of IgM and the child’s own IgG), and in seronegative children with congenital infection, IgM, IgA and IgG may appear at a later age (at 6-8 months of life); 3) enhanced antigenic stimulation of the immune system, which occurs during co-infection, especially in the case of an active viral infection (cytomegalovirus or herpetic).
The most severe consequences for the fetus and newborn occur with combined infection with Toxoplasma and CMV or HSV, and such combinations are not uncommon (it is possible to detect both intracellular organisms in alveolar and intraalveolar macrophages in a stillborn: Toxoplasma inclusions were found in the cytoplasm, and CMV in the nuclei).
Laboratory diagnosis of central nervous system lesions in CMV, HSV and EBV infections
Brain damage in the form of meningitis, menigo-encephalitis or encephalitis caused by herpes viruses occurs in people with immunodeficiencies. A high risk of the disease is observed in HIV-infected patients.
In newborns and young children, along with severe pathology of the brain, micro- or hydrocephalus can be observed; neurosonography reveals signs of hydrocephalic syndrome.
To verify the diagnosis and adequate therapy, the study of cerebrospinal fluid for the purpose of etiological decoding is of fundamental importance. In case of brain damage, it is recommended to use special test systems (manufactured by EUROIMMUN, Germany) designed to detect specific antibodies in the cerebrospinal fluid by ELISA (with parallel examination of blood serum). They contain additional calibrators for cerebrospinal fluid and allow the differentiation of antibodies produced intrathecally, from antibodies that have penetrated from the blood into the cerebrospinal fluid through the blood-brain barrier as a result of an increase in its permeability. A parallel blood serum test is required. The ratio of IgG in the cerebrospinal fluid and blood serum and the concentration of albumin in both biological fluids are determined. It is known that in case of damage to the central nervous system by toxoplasma or viruses, specific antibodies accumulate in large quantities in the cerebrospinal fluid. With local brain damage, their level in the cerebrospinal fluid significantly exceeds the content of antibodies in the blood serum. By calculating the LSQ relative index, it is possible to confirm (or exclude) brain damage by Toxoplasma or viruses. An index of more than 1.5 indicates intrathecal production of antibodies to this pathogen. With progressive brain damage, this coefficient will increase, which has important diagnostic and prognostic significance.
Due to the production of antibodies in the central nervous system under various pathological conditions (with multiple myeloma or multiple sclerosis), the Reiber diagram is used to clarify the intrathecal production of specific IgG
(1991) with the determination of the CSQ index, the calculation of which takes into account the concentration of albumin, which is produced only in the liver, but not intrathecally.
Since in the case of a mixed infection, when the blood-brain barrier is damaged, antibodies of different pathogens may appear in the cerebrospinal fluid, the use of these tests will make it possible to determine the leading agent responsible for progressive brain damage.
Interpretation of test results for cytomegalovirus infection
Specific antibodies are responsible for the lysis of the intracellular virus and also inhibit its intracellular replication or spread from cell to cell. Sera from patients after primary infection contain antibodies that react with internal proteins of CMV (p28, p65, p72). p130 is a highly specific protein. Convalescent serum contains mainly antibodies that react with membrane glycoproteins.
The detection of IgM is of greatest diagnostic importance as an indicator of the activity of the process, which may indicate an acute disease, reinfection, superinfection or reactivation. Detection of IgG in previously seronegative individuals also makes it possible to determine primary CMV infection, monitor over time individuals with clinical manifestations of infection, and provides significant assistance in retrospective diagnosis. It should be remembered that in many patients with severe immunodeficiency (including AIDS) and severe CMV infection, as well as in pregnant women and young children, the production of antibodies to CMV is slowed down. This is manifested by the detection of specific antibodies in low concentrations or the absence of positive dynamics of antibodies. In this case, the detection of IgA, “early” IgG antibodies (low-avidity antibodies) and/or “early” antigens or CMV DNA is crucial. An avidity index of less than 40% indicates the presence of primary CMV infection.
After treatment with etiotropic and immunomodulatory drugs, control tests usually show a significant increase in the level of IgG antibodies, while AI may decrease, which should be assessed as a positive factor and there should be no rush to prescribe a second course. The recent appearance of cases with negative results of control studies for CMV infection against the background of CMV persistence is alarming. This may indicate negative activation of B lymphocytes after the use of chemical immunomodulators.
Immunoblot (Westernblot) is a unique test that allows you to separately detect IgM and IgG to individual CMV proteins and monitor the dynamics of protein changes, which has a high diagnostic and prognostic value. CMV antigens are divided into three categories:
1) cross-reactive antigen with a molecular weight of 65 kDa and unidentified antigens;
2) low specific antigen with molecular masses of 55 kDa, 52 kDa and 38 kDa;
3) highly specific antigens with molecular masses of 130 kDa and 28 kDa.
The presence of highly specific antigens confirms the formation of an immune response to CMV, and the presence of at least one band of category 3 (pp 130 and p28) indicates a positive result. The presence of pp65 (immediate early protein) is regarded as a marker of primary (acute) infection or reactivation. Its accumulation during superinfection cannot be ruled out. As the infectious process progresses, p28 appears.
Experience shows the possibility of using immunoblotting both in the diagnosis of congenital CMV infection and the acquired form, including in individuals with severe immunodeficiency (with limited antibody synthesis or their polyclonal production).
The use of this test when examining children born with signs of intrauterine infection or from mothers who had clinical and/or laboratory signs of active infection during pregnancy allows us to establish (exclude) intrauterine (perinatal) infection. If during the newborn period RIF (PCR) gives negative results in a blood test, and detection of IgG (maternal antibodies) does not allow identifying CMV infection, then it is recommended to conduct a re-examination after 1.5-2 months. In case of infection, IgM antibodies to “early” proteins (p65, p28) and/or IgG to them appear, which often correlates with positive results of RIF and/or PCR and an increase in clinical symptoms (primarily from the central nervous system).
Interpretation of test results for herpes infection
Detection of DNA or early proteins of HSV-1 and HSV-2 in blood leukocytes indicates viral replication. ELISA is used to determine antibodies of the classes IgM, IgG, IgA and IgG avidity to HSV. The detection of IgM is of greatest diagnostic importance as an indicator of the activity of the process, which may indicate an acute disease, reinfection, superinfection or reactivation. However, in clinically significant cases, including the typical course of neonatal herpes, specific IgM is rarely detected. In many patients with severe immunodeficiency and severe herpes infection (genital herpes), as well as in pregnant women and young children, the production of antibodies to HSV is slowed down. This is manifested by limited synthesis of specific antibodies (they are found in low concentrations) or the absence of positive dynamics of antibodies. In this case, the identification of HSV-IgA and/or “early” HSV antigens is of critical diagnostic importance. It should be noted that an avidity index of less than 40% indicates the presence of a primary herpetic infection, however, such cases are rarely detected and occur mainly in children. Subsequently, against the background of relapses, AI most often remains at high levels, and therefore this test has lower diagnostic and prognostic information than for CMV infection.
The existing experience with test systems aimed at identifying IgA to HSV-1 and 2 has shown the advisability of its use not only for diagnostic purposes, but also for the purpose of predicting the course of the disease, assessing the effectiveness of antiviral therapy and monitoring. The test is especially important for diagnosing the congenital form of herpes infection, since IgA is one of those immunoglobulins that do not pass through the placenta, and its detection indicates infection. The appearance of IgA to HSV in the blood serum in large quantities in the presence (or absence) of clinical manifestations indirectly indicates the activation of the virus and justifies the implementation of immunorehabilitation measures even in the absence of clinical manifestations (the “preemptive therapy” method).
Immunoblot (Westernblot, Line-blot) – allows you to differentiate in one test proteins characteristic of the activation of HSV types 1 and 2, separately detect IgM and IgG to individual proteins, and monitor their changes over time, which has a high diagnostic and prognostic value. The source of antigens is the complete complex of HSV-1 type antigens and the G-2 glycoprotein of HSV-2 type, purified by affinity chromatography. Individual viral proteins of pre-cultured HSV-1 are transferred to the nitrocellulose membrane of strips using intermittent electrophoresis, then the strips are covered with a membrane chip containing purified HSV-2 glycoprotein G-2.
Specificity of antigens on the strip
| Strip on the strip | Antigen | Description | Category |
| 130 kDa | pp 130 | Phosphoprotein antigen: UL57 | 3 |
| 65 kDa | pp 65 | Tegument phosphoprotein antigen (shell protein): UL83 | 1 |
| 55 kDa | p55 | Glycoprotein B antigen gB: UL55 | 2 |
| 52 kDa | pp 52 | Phosphoprotein antigen: UL44 | 2 |
| 38 kDa | p 38 | 2 | |
| 28 kDa | p28 | Capsid protein antigen: UL99 | 3 |
Experience shows the possibility of its use both in the diagnosis of congenital and acquired herpes. This test, in combination with RIF or PCR methods, provides very valuable information for a doctor, especially when monitoring congenital and acquired forms, including genital herpes, but requires certain experience and knowledge in interpreting the results. When issuing a conclusion, it should be taken into account for what purpose and at what stage the patient is examined (for diagnostic purposes, at the observation stage, or to evaluate the effectiveness of treatment).
Interpretation of research results for Epstein-Barr virus infection
EBV is the main virus that causes in most cases an acute disease - infectious mononucleosis, manifested by fever, lymphadenopathy, inflammatory diseases of the nasopharynx, enlargement of the liver and spleen, sometimes only a rash or only fever, and characteristic changes in the hemogram in the form of a mononuclear reaction. With infectious mononucleosis, polymorphic skin rashes and damage to the central nervous system in the form of meningitis, meningoencephalitis, and encephalomyelitis are possible; Jaundice often appears. The following features of infectious mononucleosis at the present stage should be noted:
1) the number of atypical and severe forms has increased;
2) the disease has become frequently registered in young people (from 15 to 30 years);
3) cases of lymphadenopathy and low-grade fever have become more frequent, including in pregnant women, associated with EBV activation in the absence (or late appearance) of atypical mononuclear cells in the hemogram;
4) complications often began to be observed in children and young people after suffering a typical form of infectious mononucleosis in the form of persistent infection, peritonsillar abscess, furunculosis;
5) the role of EBV in the formation of pathology of the embryo (fetus) and newborn, often with damage to the central nervous system, has been proven.
Diagnosis is based on clinical, epidemiological and laboratory data. Due to the polymorphism of clinical manifestations, correct assessment of changes in peripheral blood is of great diagnostic importance. To confirm the diagnosis, various serological methods and PCR are used. Of greatest importance is the detection of DNA and/or antibodies of the IgM and (or) IgA class to the viral capsid and “early” IgG. IgM appears with the onset of the disease and persists for 1-2 months,
subsequently, synthesis switches to IgA (more often indicating reactivation). Antibodies to nuclear antigens of the IgG virus appear only 3-6 weeks after the onset of the disease and persist throughout life. Their determination is retrospective.
Detection of atypical mononuclear cells by light microscopy is the main method available to any clinical diagnostic laboratory. They usually appear from the first days of the disease and are most pronounced during the height of the disease. Sometimes they appear later. The detection of wide-plasma atypical mononuclear cells with an increase in the total number of lymphocytes is of diagnostic importance. During treatment against the background of immunomodulation in patients who did not have atypical mononuclear cells or had single ones, their appearance (increase) is possible.
ELISA is used at the first stage of the diagnostic process and for monitoring; it allows you to determine specific antibodies IgM, IgA and IgG (“early” and “late”) in blood serum. To diagnose and establish the activity of the infectious process, it is important to identify IgM, IgA and/or “early” IgG. Monitoring the dynamics of a specific immune response allows us to assess the effectiveness of the therapy. Long-term or periodically positive results for the presence of “early” IgG indicate a persistent form and possible periodic reactivation of the virus. Additional information is provided by the immunoblot method.
Immunoblot (Westernblot) – allows you to separately determine IgM and IgG to individual proteins (kits from “EUROIMMUN”, Germany), since a complete set of antigens is applied on one strip. This makes it possible to reliably identify the stage of infection, which is extremely important not only for diagnosis, but also for clinical and immunological monitoring, especially in those who have recovered from a typical form of infectious mononucleosis, taking into account the high frequency of virus persistence. Detection of VCA 125 protein indicates an early phase of infection.
Specificity of antigens on the strip
| Band | Molecular mass | Antigen | Specificity |
| gC-1 | 130 kDa | Glycoprotein C-1 (gC-1) of HSV-1 | Highly specific for herpes simplex virus type 1 |
| gB-1 | 120 kDa | Glycoprotein B-1 (gB-1) HSV-1 | Specific for herpes simplex viruses |
| gD-1 | 60 kDa | Glycoprotein D-1 (gD-1) HSV-1 | Specific for herpes simplex viruses |
| gG-2 | Glycoprotein G-2 (gG-2) HSV-2 | Highly specific for herpes simplex virus type 2 |
At the height of the disease and at the end of the acute process, VCA 19 appears. The late phase is indicated by the highly specific marker VCA 22, which is detected independently or together with EBNA-1 (p79). The latter protein is present for a long time in those who have recovered from the disease and indicates a previous infection. When conducting clinical and immunological monitoring, we showed that the absence of antibodies to p79 in children after infectious mononucleosis (severe form) correlates with the presence of DNA of this virus in scrapings from the mucous membrane of the pharynx and (or) in the leukocyte suspension and is clinically accompanied by frequent relapses of tonsillitis.
If viral DNA is detected in a leukocyte suspension or from a source of infection (scraping from the mucous membrane of the nasopharyngeal ring), it is advisable to conduct serological studies to establish the fact of a primary infection or relapse of the disease. In the presence of IgM, one can conclude about an acute infection; in the case of detection of IgA or “early” IgG, one can conclude about reactivation, and “late” proteins – about persistence.
Thus, the laboratory’s capabilities are quite sufficient for the diagnosis of herpesvirus infections, taking into account the principle of rationality and economic and clinical feasibility.
LITERATURE
1. Agadzhanova E.A., Novikova S.V., Malinovskaya V.V. and others. On the issue of diagnosis and treatment tactics in pregnant women with herpes virus infection // Russian Bulletin of Obstetrician-Gynecologist. – 2011. – No. 4. – P. 56-58.
2. Agarkova L. A., Bukharina I. Yu., Gabitova N. A. Experience in using modern algorithms for diagnosis and treatment of perinatally significant infections in women with miscarriage // Siberian Medical Journal. – 2008. – No. 4. – P. 11-14.
3. Budanov P.V., Strizhakov A.N. Etiology, pathogenesis, diagnosis and treatment of intrauterine infection. // Issues of gynecology, obstetrics and perinatology. – 2010. – T. 9, No. 3. – P. 61-71.
4. Gervazieva V. B., Samoilikov P. V. Interaction of viruses of the Herpesviridae family with the human immune system // Allergology and Immunology. – 2010. – T. 11, No. 1. – P. 31-41.
5. Dolgikh T.I. Laboratory diagnostics is the basis for information support of the diagnostic process for opportunistic infections // Clinical laboratory diagnostics. – 2008. – No. 1. – P. 49 – 51.
6. Dolgikh T.I., Shelev M.V., Minakova E.Yu. Modern strategy for laboratory diagnosis of herpesvirus infections // Modern laboratory diagnostics. – 2011. – No. 3. – P. 19-22.
7. Kudin A.P., Germanenko I.G., Astapov A.A. The role of Herpes simplex in human pathology // Medical news. – 2004. – No. 5. – P. 11-14.
8. Podkolzova N.M., Skvortsova M.Yu., Melnikova N.I., Ostreykov I.F. Intrauterine infection: current state of the problem // Obstetrics and gynecology. – 2009. – No. 3. – P. 27-32.
9. Anzivino E., Fioriti D., Mischitelli M. et al. Herpes Simplex Virus Infections in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention //Virology J. – 2009. – 40 (6). – P. 1-11.
10. Cimolai N., Thomas EE, Tan R., Hill A. Utilization of herpes simplex PSA assays for cerebrospinal fluid in a pediatric health care setting. //Can. J. Microbiol. – 2001. – 47 (5). – P. 392-396.
11. Kimberlin David W. Herpes Simplex Virus Infections of Newborn // Curr.
Probl. Pediatr. Adolesc Health Care. – 2009. – No. 39. – R. 7-23.
How is the disease transmitted?
You need to know this in order to correctly prescribe a diagnosis. There are many ways of transmission. The virus can be located on any part of the skin or mucous membrane, that is, where there are epithelial cells.
The most common ways of transmitting the human papillomavirus are:
- Sexual vaginal contact.
- Anal sex.
- Oral sex (including cunnilingus) - possible infection of the pharynx and larynx with the development of oncology of these organs.
- Kiss in the presence of microtraumas of the mucous membrane.
- From mother to fetus as it passes through the birth canal.
- In case of close contact of damaged skin with papillomas of another person. The lesions may not be noticeable and often form during sexual contact in the pubic area, scrotum, and labia, which makes it possible to become infected with HPV when using a condom.
- Transfer of the virus from the surface of warts to damaged skin.
Papillomavirus 16/18 (qualitative method)
Human papillomavirus infection is the general name for a number of viral diseases, a characteristic feature of which is the formation of wart-like growths on the surface of the skin and mucous membranes. Infections of this series are chronic, with periodic relapses, highly contagious and widespread. The disease is caused by the human papillomavirus (HPV), which has more than 100 genotypes known to medicine. Infection with the virus usually occurs through sexual intercourse, but there are known cases of infection of children from sick mothers during childbirth.
The human papillomavirus does not always make itself felt in a visible way. There are known cases of complete spontaneous disappearance of the virus from the body (this is especially true for young people). However, long-term localization of the infectious agent in the tissue covering the surface of the uterus (epithelium) is fraught with the occurrence of abnormal changes.
Diseases caused by the papilloma virus are varied and depend on its type. The consequence of HPV infection at a certain stage may be damage to the middle layer of the epithelium, characterized by the formation of a scaly transformation of cervical cells. The occurrence of the third stage of this condition becomes possible only with the constant presence of an infectious agent belonging to a genotype with a high oncological risk. There is evidence that the presence of such genotypes for 5-10 years in the body of women over 30 years of age significantly increases the risk of cervical cancer. According to statistics, more than 90% of cases of cervical cancer are characterized by the presence of the papilloma virus. The 16th and 18th types are the most common of the detected types. Their detection during examination of a sample does not yet mean the presence of cancer, but serves as a signal for further examinations and monitoring of the behavior of the infection.
The consequence of infection with the human papillomavirus can be not only the formation of a cancerous tumor of the female genital organs, but also the development of squamous cell carcinoma in representatives of both sexes. Also, studies have proven the direct involvement of the virus in education:
- condylomas - formations that are the result of growth of the skin or mucous membrane in the form of a papilla in places of irritation, friction, incl. in the genital area;
- genital warts (varieties of condylomas) - small growths that are body-colored and form on the genitals, around the anus, and occasionally in the oral cavity.
This testing is designed to detect specific DNA fragments of human papillomavirus types 16 and 18. The study is carried out using the polymerase chain reaction method, the essence of which is to significantly increase the number of HPV-specific viral DNA fragments with monitoring the process in real time. The material selected for the study is cells of epithelial tissue of the urogenital tract.
Obtaining a correct research result is only possible if the sampling of epithelial cells of the urogenital tract is carried out strictly in accordance with technical requirements. This can be determined by an additional indicator - KVM (material sampling control), which allows you to find out how many specific fragments of viral DNA were included in the sample with epithelial cells.
Analytical indicators:
- test area: DNA fragments characteristic of HPV types 16, 18;
- test specificity – 100%;
- detection sensitivity for the above types of human papillomavirus is 100 copies of DNA in the test sample.
Virus prevention
It is possible to prevent both primary infection and infection of a healthy sexual partner, as well as recurrence of human papillomavirus infection.
To prevent the virus from entering during sexual intercourse, it is necessary to use a condom and avoid contact of the skin of a healthy person with areas where there are papillomas in an infected person.
Timely removal of manifestations of papillomavirus infection - papillomas and condylomas - will help reduce the risk of infection. This, of course, does not completely get rid of HPV, but it reduces its amount on the surface of the skin. Antiviral and immunomodulatory therapy directed against the human papillomavirus can also help.
In adolescents who have not had sexual contact and do not have genital types of HPV, a special vaccine is used for prevention, which allows the immune system to produce antibodies against the virus, which prevents it from fixing in cells if infection occurs in the future. This reduces the risk of cervical cancer, which is very often associated with papillomavirus.
Prevention of skin papillomas can be achieved by timely treatment of scratches, abrasions, abrasions, calluses and other skin lesions with antiseptics, which allows you to close the entrance gate to the virus during tactile contact with a sick person.
Papillomavirus genotyping (16,18,31,33,35;39,45,51,52,56,58,59) (qualitative method)
Human papillomavirus infection is the general name for a number of viral diseases, a characteristic feature of which is the formation of wart-like growths on the surface of the skin and mucous membranes. Infections of this series are chronic, with periodic relapses, highly contagious and widespread. The disease is caused by the human papillomavirus (HPV), which has more than 100 genotypes known to medicine. Infection with the virus usually occurs through sexual intercourse, but there are known cases of infection of children from sick mothers during childbirth, as well as transmission of the infection through household contacts.
The most likely infection with human papillomavirus infection is:
- with early onset of sexual activity;
- with a large number of sexual partners;
- with low immune defense;
- when using oral contraceptives;
- with vitamin deficiency;
- in the presence of sexually transmitted infections;
- with an addiction to smoking;
- when living in big cities.
The dependence of the probability of infection on the number of sexual partners can be illustrated by the following observational data: among women with one partner, the number of people infected with the virus is 17-20%, with five or more partners - about 70-80%.
The incubation period for HPV can vary from 2 months to 10 years. Typically, the infection is asymptomatic. Even colposcopic, cytological and histological examinations may show normality. The latent form of infection with the human papillomavirus can only be detected using the PCR method.
In almost a third of patients, the infection spontaneously disappears from the body within six months to a year (this is especially true for young people).
Diseases caused by the papilloma virus are varied and depend on its type. The consequence of HPV infection at a certain stage may be damage to the middle layer of the epithelium, characterized by the formation of a scaly transformation of cervical cells. The development of the third stage of this condition becomes possible only with the constant presence of an infectious agent belonging to a genotype with a high oncological risk. There is evidence that the presence of such genotypes for 5-10 years in the body of women over 30 years of age significantly increases the risk of cervical cancer. HPV infection of trophoblast cells (the outer layer of embryonic cells) can lead to spontaneous abortion.
The consequence of HPV infection can be not only the formation of a cancerous tumor of the female genital organs, but also the development of squamous cell carcinoma in representatives of both sexes. Also, studies have proven the direct involvement of the virus in the emergence of:
- condylomas - formations that are the result of growth of the skin or mucous membrane in the form of a papilla in places of irritation, friction, incl. in the genital area;
- genital warts (varieties of condylomas) - small growths that are body-colored and form on the genitals, around the anus, and occasionally in the oral cavity;
- warts
These formations are benign and appear during acute infection. Infection with HPV in children is fraught with the development of laryngeal papillomatosis.
The cause of infection in men in 40-60% of cases is sexual contact with an infected woman. During the study, they are found to have the same HPV genotypes as their partners. Papillomavirus in males can manifest itself as characteristic rashes on the skin and mucous membranes of the genital organs.
This testing is designed to detect specific DNA fragments 16, 18, 31, 33, 35; 39, 45, 51, 52, 56, 58, 59 types of human papillomavirus. The study is carried out using the polymerase chain reaction (PCR) method, the essence of which is to significantly increase the number of HPV-specific viral DNA fragments with monitoring the process in real time. The material selected for the study is epithelial cells of the urogenital tract.
The identification of human papillomavirus genotypes with a high oncological risk during the study does not mean the presence of cancer, but is an indication for additional histological and cytological studies and monitoring the development of the infection over time.
The importance of repeated genotyping is due to the following arguments:
1. The presence of several genotypes of the human papillomavirus in the body is an indicator of the likelihood of a more severe infection with a higher risk of maintaining the virus in an active state;
2. Different genotypes are characterized by varying degrees of carcinogenic activity. The most dangerous are HPV types 16 and 18. If specific fragments of HPV DNA are detected after testing for a wide range of types, it is necessary to conduct a test to identify 16 and 18 genotypes. Confirmation of their presence is an indication for colposcopy. If, upon re-examination, other genotypes of high cancer risk are detected, cytological examinations are recommended, and then, if a positive answer is obtained, colposcopy.
3. Repeated genotyping makes it possible to determine what exactly is taking place: reinfection or persistent (chronic) infection (in the first case, the spectrum of genotypes changes, in the second, the genotype remains the same). This is important to know because the chronic form poses a significant risk, while a recent infection may resolve spontaneously. In addition, re-infection with the same genotype after spontaneous recovery is almost impossible.
Obtaining a correct research result is only possible if the sampling of epithelial cells of the urogenital tract is carried out strictly in accordance with technical requirements. This can be determined by an additional indicator - KVM (material sampling control), which allows you to find out how many specific fragments of viral DNA were included in the sample with epithelial cells.
Analytical indicators:
- test area: DNA fragments characteristic of HPV types 16, 18;
- test specificity – 100%;
- detection sensitivity for the above types of human papillomavirus is 100 copies of DNA in the test sample.
Get tested for HPV in Moscow with us
To plan preventive methods, of course, HPV diagnosis is important.
You need to know whether you have this virus or not in order to understand the risks for sexual partners and the prospects for the development of this disease. Therefore, even if there are no manifestations, tests for papillomaviruses are necessary during a full examination by a urologist or gynecologist. Where you can get tested for HPV: daily from 10:00 to 21:00 in our clinics, seven days a week and on holidays. If necessary, you can get comprehensive advice on the diagnosis and treatment of human papillomavirus infection from experienced urologists, gynecologists and venereologists.