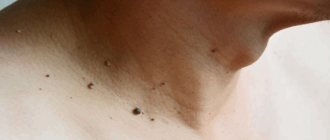
Discharge from the mammary gland
When pressing on the nipple, a woman may have different discharge patterns. Sometimes they spontaneously leave stains on laundry.
If this discharge is the same on the right and left , it is never associated with a tumor or papilloma. Most likely, such discharge is associated with hormonal changes or incorrectly selected hormonal medications (which the woman is taking).
Discharge from nipples
These photos show light or slightly cloudy scanty whitish discharge - they are not a pathology . Moreover, their volume and color are the same on the right and left . Light transparent, or brown, yellow discharge is not a manifestation of papilloma.
If they are symmetrical on the right and left in volume and color but abundant , they are most often associated with hormonal changes (ovaries or thyroid gland).
With papilloma, the discharge is always asymmetrical: abundant and on one side.
Diagnostics
In order to recognize the condyloma virus or papilloma, it is necessary, first of all, to consult a highly qualified doctor. Treatment of diseases on the body can be carried out by a dermatologist or cosmetologist, on the male genital organs by a urologist, on female genitals by a gynecologist, and near the anus by a proctologist.
In order to accurately establish the diagnosis, the doctor conducts a full examination of the patient at the first appointment. This is necessary in order to distinguish genital warts from other diseases similar to it in appearance.
Specialists at our clinic examine all patients with genital warts for the presence of HIV diseases and syphilis. If necessary, examination is also carried out for the presence of other diseases transmitted during sexual intercourse. In this case, genital warts are removed using the most convenient and accessible method.
Blood from the gland
Photo of bloody discharge from the nipple of the mammary gland and ultrasound marking of the dilated duct with papilloma (first photo). Such markings help during surgery to quickly and accurately detect the fragment of the mammary gland that needs to be removed.
Blood from nipple video
Video of bloody discharge from the nipple with pressure on the duct marked by ultrasound.
This is how this papilloma looks on an ultrasound and this is how the ultrasound doctor who performed the markings before the operation on the same patient describes it in detail:
| Focal changes in structure: LEFT GLAND At a distance of 50-75 mm from the center of the nipple in the lower-outer quadrant, at a depth of 15.6 to 20.1 mm, a duct dilated for up to 40 mm with a parietal tissue inclusion of 9.4x2.9x4.5 mm is determined - cannot be excluded intraductal papilloma 9.4x2.9x4.5mm v=0.064cm3 NB marks were made with a pencil “Edding 8020 haut-skin marker” in a supine position, lying down, arms raised behind the head. |
Discharge from the nipples of the mammary glands
Pus from the nipple is not actually pus, but just a discharge. If it were pus, there would be fever, pain, redness of the skin - signs of inflammation. More often, such discharge occurs with ductectasia (dilation of the ducts, which can be detected by ultrasound) and with hormonal changes.
This is not a global problem and does not lead to cancer, but it is recommended that such discharge be applied to a glass slide and examined under a microscope (cytological analysis of discharge).
Nipple discharge during
Nipple discharge may occur during orgasm. This is due to muscle contraction and squeezing out contents from the ducts. Another thing is why there is so much of this content that it does not remain in the ducts, but is squeezed out. More often this is due to hormonal changes (gynecology, thyroid gland or excess intake of hormones from food - broiler poultry, fish, semi-finished broiler meat products).
Are breast papillomas dangerous during pregnancy?
Regardless of location, papillomas during pregnancy can cause significant discomfort to a woman and cause concern for the health of the child. It should be understood that the papillomavirus cannot influence the development of the baby in any way, however, with a high degree of probability, the child will be a carrier of the viral agent at birth, which will enter the body through the perinatal route of infection. This most often manifests itself in the formation of epidermal neoplasms in childhood.
As for the risks to a woman’s health, there are several potential dangers, the most significant of which can be considered the possibility of malignant transformation of a harmless neoplasm, which leads to an oncological process. This is especially true for intraductal papillomas, which can exist for a long time in a latent, undiagnosed form. In addition, such papillomas after childbirth during the process of active growth can clog the duct, preventing the outflow of breast milk, which can cause stagnation.
There is also a risk of mechanical damage to the tumor when wearing too tight underwear and clothes. This outcome may be accompanied by severe bleeding and infection of the wound, which is unacceptable during pregnancy.
Discharge from the nipple of the breast
light serous or white discharge with ductal papilloma , but then it is abundant and more pronounced only on one side (not symmetrically).
Then it is necessary to analyze these secretions under a microscope for papilloma cells, ultrasound for detection of ductal papilloma and/or ductography for the same purpose. Please note that it makes sense to do these studies only with those who already have experience working with this problem, and not with the first doctor you come across.
Nipple discharge
A clear sign of breast duct papilloma is bloody discharge from the nipple when pressed or spontaneous - like stains on linen.
Data allocation is also not a problem. Most likely, they are also associated with hormonal changes, and the dark drop from one duct is due to ductectasia (dilation of the duct after feeding). An examination of the secretions under a microscope is shown. If red blood cells or papilloma cells are found - ultrasound and ductography, with the prospect of surgery.
Brown discharge from the nipple
Brown or rusty discharge from the breast ducts - may appear as stains on laundry. The rusty color is due to destroyed red blood cells. This is also a sign of bleeding.
What not to do?
Doctors do not recommend removing hanging moles and papillomas on the nipples during pregnancy. Carrying a child is one of the factors that provokes the development of neoplasms, since during this period a complete restructuring of the hormonal background occurs. It is likely that after childbirth, when hormone levels return to normal, the papillomas will disappear on their own, without surgery.
If this does not happen immediately after birth, you should definitely consult a doctor. Papillomas can make breastfeeding difficult; in addition, with microtraumas, the risk of infecting the child with the virus increases, if this did not happen during pregnancy or childbirth.
It is strictly contraindicated to treat breast formations with folk remedies on your own.
Blood from the nipple
Blood discharge from the nipple ducts must be distinguished from nipple cancer. With nipple cancer, there may also be blood discharge, but then a wound with crusts appears on the skin of the nipple. Signs of nipple cancer SEE HERE .
Photo of a nipple with bloody discharge due to ductal papilloma of the mammary gland (cystadenopapilloma).
This kind of bloody discharge from the nipple should alert you! With them, it is necessary to analyze them under a microscope for papilloma or cancer cells. Detection of red blood cells in the analysis is a potential indication for surgery - removal of all ducts or a duct for its examination.
Papillomas during pregnancy: treatment
It is advisable to remove any neoplasms located on the skin and mucous membranes before pregnancy. If for any reason this rule was not followed, or primary papillomas appeared during pregnancy, the woman should immediately contact a specialist. Depending on the location of the defect, medical advice and assistance can be provided by a dermatologist, mammologist, gynecologist or oncologist.
It is strictly forbidden to resort to self-medication with the help of pharmaceutical ointments and plasters, folk recipes, as well as mechanical cutting of the body of the wart during pregnancy.
Intraductal papilloma of the mammary gland on ultrasound
In practice, we often have to console frightened patients whose ultrasound revealed “ductal papilloma”, but presented completely unclear and dubious ultrasound images, did not confirm it with an analysis of nipple discharge, did not do mammography (for a girl over 35 years old) or ductography, but offered it immediate VAB removal. Obviously, this approach is justified only for making money on the patient’s fear.
In the photo, a 37-year-old patient has abundant light transparent discharge from the nipple of the right mammary gland (taken for analysis under a microscope), ultrasound revealed a papilloma in the duct, mammography with ductography in 2 projections contrasted the ductal system with a filling defect - due to for papillomas located in this place. After ductography, the amount of discharge temporarily decreased (the contrast has a cauterizing effect). Planning to give birth. Therefore, we proposed removal of only the duct with papilloma, and not all ducts (Babcock's operation - selective ductectomy).Human papillomavirus infection and pregnancy. Features of diagnosis and management tactics
The prevalence of human papillomavirus infection (PVI) and, accordingly, the pathology associated with it has been steadily increasing over the past decades in many countries of the world, including Russia. According to the literature, up to 3 million new cases of human papillomavirus (HPV) infection are registered annually in the world [1]. Transmission of HPV from person to person can occur in several ways: household contact, vertical, genital, oral, anogenital contact. The high increase in HPV infection in the population, due to its significant contagiousness, the variety of pathologies associated with it, and, most importantly, the ability to transform epithelial cells, triggering the process of carcinogenesis, attract the attention of various specialists to the search for treatment options, timely diagnosis and prevention of diseases associated with HPV [2, 3]. In this case, special attention is paid to PVI of the urogenital tract, which occupies a leading position in prevalence among sexually transmitted infections. The development activity and formation of HPV-associated, as well as other infectious pathologies, are largely determined by the state of the immune system and its ability to adequately respond to the presence of a pathogen. At the same time, HPV, by blocking certain parts of the immune system and exhibiting resistance to TNF-mediated inhibition of proliferation, which is associated with a significant decrease in the expression of TNF receptors, is able to “escape” the immunological surveillance system [4–6]. It has been established that HPV, due to the activation of ubiquitin-mediated proteolysis of the p53 protein, which is a suppressor of carcinogenesis, is able to influence the mechanisms of regulation of the molecular genetic cycle of cell division and block apoptosis [7–9]. It has also been proven that the HPV E7 protein neutralizes the antiviral and antitumor activity of interferon-α2 due to its ability to selectively block most genes induced by interferon [10]. Such multidirectional mechanisms of interaction between HPV and the human immune system contribute to survival, long-term persistence of the virus and a high risk of developing HPV-associated pathology, especially in the presence of trigger factors.
The consistent and holistic picture of the epidemiology and pathogenesis of PVI that has emerged over the past two decades is more clearly presented in women than in men [11]. PVI is registered in 40%, 70% and more than 90% of cases of vulvar, vaginal and cervical cancer, respectively, which are the second leading cause of death in women worldwide [11–14]. Recently, about 20 types of HPV have been associated with cervical canal cancer (95%), among which the most frequently detected are HPV type 16 (50%) and HPV type 18 (10%) [15]. It should be noted that in addition to HPV, a number of associated factors play a determining role in the development of oncogenic transformation. Here, first of all, concomitant infectious diseases of the anogenital area should be highlighted. The combined persistence of HPV with HSV type 2, CMV, EBV, HIV, chlamydia and mycoplasma is unfavorable, especially in terms of the development of cervical dysplasia. Of particular importance, as mentioned above, in the occurrence of infection, the severity of its course, outcome, quality and control of the treatment process for patients with pathologies of the skin and urogenital tract caused by HPV, is the nature of the immune response [16].
One of the features of HPV infection of the urogenital tract is considered to be its wide distribution among young women of reproductive age, mainly under 25 years of age [17], which is due to the low sexual culture of the population, frequent changes of sexual partners, unprotected sex, bad habits (smoking, substance abuse, alcoholism) . There has been an increase in the incidence of anogenital warts in prepubertal children and adolescents, which can be partly explained by an increase in the number of children who become sexually active early. According to sociological surveys, about 15% of girls and 22% of boys noted the presence of sexual contacts in their lives, while 50% of them indicated that their first sexual contact occurred before the age of 15, and for 5% of girls and 20% of boys - up to 12 years [16]. At the same time, it has been noted that spontaneous elimination of HPV occurs more often and faster in adolescents and young women (up to 80% of cases) and regression of existing HPV-associated pathology compared to women of later age. Studies have shown that the average time to clear HPV in adolescents is 8 months (CDC, 1999). According to the observations of S.I. Rogovskaya and V.N. Prilepskaya (2006), in every second patient aged 18–25 years, this period increases to 1.5–2 years.
HPV infection becomes particularly relevant during pregnancy, with the frequency of registration of all types of HPV in pregnant women being 30–65%, and high oncogenic risk types being 20–30% [18].
The main feature of pregnancy is that the fetus in relation to the mother is genetically a half-alien (semi-allogeneic) organism, which is not rejected before the due date. The allogeneity of the fetus lies in the fact that all cells contain, in addition to the haploid set of HLA antigens of the mother, the haploid set of HLA antigens of the father. The maturation of a fertilized egg into a mature fetus in the half-alien body of the mother is carried out due to a suppressor mechanism that develops from the first hours after conception and operates until the development of labor. This mechanism does not allow the mother’s immune system to carry out an immune attack on the fetus with the aim of rejection at all stages of its development [19].
The suppression that develops after conception is multifactorial and is formed both due to products of the endocrine system and due to certain changes in systemic and local immune reactions developed in the process of evolution to protect the semi-allogeneic fetus from the mother’s immune system: the absence of classical antigens of the HLA class I and II; a shift in the functional balance of T helper cells towards type 2 cells and the immunoregulatory role of the placenta, providing a unique immunosuppressive background in the mother’s body [20].
Thus, fertilization itself is of an immune nature. Active processes aimed at local immunosuppression are carried out throughout pregnancy in the fetoplacental complex.
In such a situation, PVI is not only a high risk of developing HPV-associated pathology against the background of physiological immunodeficiency, but also the possibility of its transmission from mother to child during childbirth [21]. In 1989, vertical transmission of the virus was proven, which is confirmed by reports of the detection of HPV in the amniotic fluid of pregnant women and in children born to mothers who are HPV carriers [22]. The possible risk varies according to different authors from 3% to 80% [23]. This scatter is explained by differences in the polymerase chain reaction (PCR) method for detecting HPV DNA. In this case, PVI can be transmitted transplacentally and intranatally (in particular, HPV types 6 and 11). The risk of infection is directly proportional to the severity of the infection (the number of viral particles) and the time of the anhydrous interval during labor, however, studies indicate that delivery by cesarean section does not reduce the risk of infection of the fetus, which indicates predominantly intrauterine infection [22]. Intrapartum infection can lead to juvenile recurrent respiratory papillomatosis (incidence is 1.7–2.6 per 100,000 children and 1 per 1,500 births among women with genital PVI) [24].
Invasion of the virus occurs through microdamage to the skin and mucous membranes with infection of predominantly immature, dividing cells of the basal layer of the epithelium, which are target cells for HPV. Next, the virus replicates and assembles viral particles in differentiated cells of the surface layer of the epithelium. In this case, HPV can have a productive or transformative effect on the epithelium. With productive exposure, benign neoplasms arise - papillomas, warts and condylomas of the skin and mucous membranes. The result of the transformative effect is dysplasia of varying severity, the progressive development of which leads to cancer [25, 26]. Strong evidence of the acceleration of the development of anogenital cancer and an increase in cancer risk with early exposure to PVI emphasizes the particular importance of not only studying the prevalence of PVI in children, but, most importantly, regular and long-term monitoring of them, taking into account the possibility of developing anogenital neoplasia [27].
There are studies in the literature proving that with PCR-confirmed PVI in the mother, structural damage to the components of the placenta at the end of pregnancy is determined in 76.8% of cases and occurs with morphofunctional signs of chronic placental insufficiency, fetal malnutrition and complications of the neonatal period [28, 29].
Currently, there is an increase and growth of laryngeal papillomatosis in both adults and children. At the same time, transmission of infection prevails in adults through oroanogenital contacts, and in children through passage through the infected birth canal and through household contact.
The manifestation of juvenile respiratory papillomatosis in 75–85% of cases is recorded in the first 5 years of life; in 5–6% of cases in children from 6 months to a year; in 45% of cases in children under 3 years of age [28].
The clinical picture of respiratory papillomatosis consists of voice and breathing disturbances. Most often, when the larynx is damaged in the area of the commissure and the anterior parts of the vocal folds, hoarseness of the voice develops, up to its complete loss. As the lumen of the larynx narrows with papillomas, stenosis develops, and death from asphyxia is possible. The pathological process in childhood is active, it is characterized by prevalence and recurrence rate, and therefore children undergo multiple surgical interventions to remove papillomas. Repeated repeated excision of laryngeal tumors leads to the development of scar complications, the need for tracheostomy, loss of the ability to speak, and worsening chronic respiratory hypoxemia. As the tumor progresses and spreads into the distal respiratory tract, the disease is often fatal [24].
Considering that infection of a child with PVI with the development of juvenile recurrent respiratory papillomatosis is possible not only in the intranatal, but also in the postnatal period, as well as the frequent registration of HPV-associated pathology in newborns and young children, patients should be more actively and thoroughly examined at the planning stage pregnancy and during pregnancy. At the same time, attention should be paid not only to examination for highly oncogenic types of HPV, but also low-oncogenic types, taking into account that, as a rule, in patients suffering from recurrent respiratory papillomatosis, low-oncogenic HPV types 6 and/or 11 are more often found, and they are also often registered and for anogenital warts and genital warts on other areas of the skin and mucous membranes.
The purpose of the study was to assess the frequency of HPV-associated pathology of the skin and mucous membranes of non-genital localization in pregnant women with an established diagnosis of anogenital warts.
Material and research methods
We observed 76 pregnant women (gestational age from 12 to 26 weeks) aged 18 to 42 years with an established clinical diagnosis of anogenital (venereal) warts. The average age of the patients was 26.3 years. In 53 (69.7%) patients, the duration of the disease ranged from 3 weeks to 6 months, in 23 (30.3%) patients - from 6 months to 1 year. 18 (23.7%) patients reported relapses of anogenital warts after previous treatment with destruction methods (laser vaporization, electrosurgical excision, cryodestruction). All patients were referred from antenatal clinics for removal of anogenital warts. Of these, only 2 (2.6%) patients had a combination of genital warts of anogenital and extragenital localization. In 42 (55.3%) patients included in the study, PVI was confirmed by identifying HPV using real-time polymerase chain reaction (RT-PCR): HPV DNA types 16, 31, 33, 35, 52, 58 were identified in 18 (42.9%) patients, HPV types 18, 39, 45, 59 - in 14 (33.3%), HPV types 51, 56 - in 10 (23.8%); Only 6 (14.3%) patients were examined for HPV of the low oncogenic type: types 6 and 11 were found in 5 of them (11.9%). HPV identification by RT-PCR or other laboratory method was not carried out in 34 (44.7%) patients. Results of the study: during a physical examination of the patients at the time they sought medical help, 39 (51.3%) patients were found to have genital warts on the skin and mucous membranes of the extragenital areas: in 14 (35.9%) on the skin of the nipple and peripapillary area; in 11 (28.2%) on the skin of the extremities; in 9 (23.1%) - on the skin of the navel; in 5 (12.8%) - on the oral mucosa. In the anogenital area, papillomatous growths were recorded on the skin of the labia majora - in 5 (6.6%), on the mucous membrane of the vulva - in 30 (39.5%), in the area of the posterior commissure - in 4 (5.3%), on the mucous membrane the membrane of the external opening of the urethra - in 2 (2.6%), on the skin of the perianal and inguinal areas - in 4 (5.3%), on the vaginal mucosa - in 48 (63.2%) patients. Combined damage to several anatomical zones of the anogenital region was observed in 15 (19.7%) patients.
All patients underwent removal of genital warts using the radio wave method. According to our observations, the optimal pregnancy period for this procedure is determined to be after 16 weeks of gestation, when the main stage of placenta formation ends and the immunosuppressive background in the mother’s body is significantly reduced, which sharply reduces the risk of possible relapses.
Thus, the frequent detection of combined HPV-associated pathology of the anogenital and extragenital areas in pregnant women indicates the need for a more thorough examination of such patients in order not only to minimize the risk of complications and recurrence of HPV-associated pathology, but also to exclude infection of the child. A more in-depth examination of pregnant women for HPV, including not only high-oncogenic but also low-oncogenic types, will make it possible to avoid or significantly reduce the risk of developing HPV-associated pathology in children.
Literature
- Nyitray AG, Iannacone MR The epidemiology of human papillomaviruses // Curr Probl Dermatol. 2014; 45(1):75–91.
- Semenov D. M., Danko S. N., Dmitrachenko T. I. Human papillomavirus infection (clinical and pathogenetic features, treatment, prevention). Educational and methodological manual. Vitibsk: State. honey. univ. SPb: Dialect. 2008. 84 p.
- Kiselev V.I. Human papillomaviruses in the development of cervical cancer. M.: , 2004. 180 p.
- Rakhmatullina M. R., Nechaeva I. A., Shalva Mardi. Experience of destructive therapy of anogenital warts // Bulletin of Dermatology and Venereology. 2016. No. 5. pp. 96–101.
- Tavares MC, de Lima Junior SF, Coelho AV et al. // Ann Hum Biol. 2015, Jun; 16; 1–8.
- Prabhavathy D., Subramanian CK, Karunagaran D. Re-expression of HPV16 E2 in SiHa (human cervical cancer) cell potentiates NF-kB activation induced by TNF-α concurrently increasing senescence and survival // Biosci Rep. 2015, Feb; 25; 35(1).
- Aguilar-Martinez E., Morrisroe C., Sharrocks AD The ubiquitin ligase UBE3 A dampens ERK pathway signaling in HPV E6 transformed HeLa cells // PLoS One. 2015. Mar; 27; 10 (3).
- Holloway A., Simmonds M., Azad A., Fox JL, Storey A. Resistance to UV-induced apoptosis by β-HPV5 E6 involves targeting of activated BAK for proteolysis by recruitment of the HERC1 ubiquitin ligase // Int J Cancer. 2015, Jun; 15; 136(12):2831–2843.
- Jing K., Shin S., Jeong S., Kim S., Song KS, Park JH, Heo JY, Seo KS, Park SK, Kweon GR, Wu T., Park JI, Lim K. Docosahexaenoic acid induces the degradation of HPV E6/E7 oncoproteins by activating the ubiquitin-proteasome system // Cell Death Dis. 2014, Nov; 13; 5:1524.
- Sukhikh G. T., Prilepskaya V. N., Rogovskaya S. I. et al. The use of interferon drugs in the treatment of low-grade squamous intraepithelial lesions of the cervix // Effective pharmacotherapy in obstetrics and gynecology. 2009. No. 4. pp. 36–41.
- Batyrshina S.V., Shulaev A.V., Akberova D.R. Human papillomavirus infection: optimization of diagnosis and treatment // Clinical dermatology and venereology. 2015. 14 (5). pp. 67–77.
- Baldwin A., Pirisi L., Creek KE NFI-Ski interactions mediate transforming growth factor beta modulation of human papillomavirus type 16 early gene expression // J Virol. 2004, Apr 1; 78(8):3953–3964.
- Byg L.M., Vidlund J., Vasiljevic N. et al. NF-kB signaling is attenuated by the E7 protein from cutaneous human papillomaviruses // Virus Res. 2012, Oct 1; 169(1):48–53.
- Grulich AE, Jin F, Conway EL et al. Cancers attributable to human papillomavirus infection // Sex Heath. 2010; 7 (3): 244–252.
- Danilova O. V., Yunusova E. I. Tactics for managing papillomavirus lesions of the genitals. Training manual for doctors. Kazan, 2012. 30 p.
- Yunusova E. I., Danilova O. V., Gizatullina R. D. HPV-associated pathology of the urogenital tract. Collection of materials of the V All-Russian scientific and practical conference with international participation “Kazan dermatological readings: synthesis of science and practice. Kazan, 2022. pp. 117–121.
- Van Krogh D., Lacey S.D., Gross D., Baracco R., Schneider A. European guidelines for anogenital warts // STIs. 2002. No. 3. P. 29–37.
- Szepietowska M., Sfodzifski H. et al. Evaluation of frequency of HPV infection during pregnancy // Ginecol Pol. 2002; 73(8):662–665.
- Dolgushina N.V. Immunological aspects of the development of placental insufficiency and miscarriage in patients with chronic viral infections // Obstetrics and Gynecology. 2008. No. 4. pp. 16–19.
- Wicherek L., Basta P., Sikora J. at al. RCAS1 decidual immunoreactivity in servere pre-eclampsia: Immune cell pre-cence and activity // Amer. J.Period. Immunol. 2007. V. 68, No. 4. P. 358–366.
- Woodhall UK, Jeet M, Soldan K et al. Effect of genital warts: the loss of quality of life and cost of treatment in eight sex clinics in the UK // Sex and the transmissions. 2011; 87:458–463.
- Sedlacek TV, Lindheim S. et al. Mechanism for HPV transmission at birth // Am J Obstet Gynecol. 1989; 161:55–59.
- Watts DH, Koutsky LA et al. Low - risk perinatal transmission of HPV: results from a prospective cohort study // Am J Obstet Gynecol. 1989; 178:365–373.
- Green GE, Bauman NM, Smith RJ Pathogenesis and treatment of juvenile onset recurrent respiratory papillomatosis // Otolaryngol Clin North Am. 2000; 33(1):187–207.
- Rogovskaya S.I. Human papillomavirus infection in women and cervical pathology. M.: GEOTAR-Media, 2005. pp. 15–17.
- Yunusova E.I., Yusupova L.A., Mavlyutova G.I., Garayeva Z.Sh. Flat warts: features and treatment options // Treating Doctor. 2016. No. 5. pp. 52–55.
- Kokolina V.F., Malinovskaya V.V. Human papillomavirus infection. A manual for doctors. M., 2008. 44 p.
- Vorobtsova I.N., Tapilskaya N.I., Gaidukov S.N. Results of examination of newborns born to mothers with various forms of human papillomavirus infection // Pediatrician. 2011. T. II. No. 4. pp. 72–75.
- Chistyakov M.A. Pathomorphology of human papillomavirus infection in the “mother-placenta-fetus” system. Author's abstract. dis. ... Ph.D. M., 2008. 25 p.
E. I. Yunusova1, Candidate of Medical Sciences O. V. Danilova, Candidate of Medical Sciences L. A. Yusupova, Doctor of Medical Sciences, Professor G. I. Mavlyutova, Candidate of Medical Sciences Z. Sh. Garayeva, Candidate of Medical Sciences
GBOU DPO KSMA Ministry of Health of the Russian Federation, Kazan
1 Contact information
Human papillomavirus infection and pregnancy. Features of diagnosis and management tactics / E. I. Yunusova, O. V. Danilova, L. A. Yusupova, G. I. Mavlyutova, Z. Sh. Garayeva For citation: Attending physician No. 5/2018; Page numbers in the issue: 56-59 Tags: human papillomavirus infection, skin lesions, sexual transmission
Breast papilloma surgery
Treatment of cystadenopapilloma is surgical. The operation consists of removing all the ducts of the mammary gland (Koenig operation or ductectomy). The patient will not be able to breastfeed after this operation. Such an operation is possible under a compulsory medical insurance or voluntary medical insurance policy.
For nulliparous women, or in cases where our patient plans to give birth and breastfeeding in the future, we offer Babcock surgery - this is when we find, isolate and remove only the duct with papilloma . In this way, it is possible to preserve the possibility of lactation in a woman through other (unremoved) ducts of this mammary gland.
Photo of the discharge of a duct with papilloma during Babcock's operation: the duct filled with bloody contents is different from all the others, which remain intact and will allow the girl to breastfeed in the future.
Video of the isolation of a blood-filled duct with papilloma during Babcock's operation: the remaining ducts - the light ones - remain intact, and the girl will be able to breastfeed.
And what danger can neoplasms on the breast pose?
The female body is an amazing biological system, which during pregnancy faces enormous stress and not only successfully overcomes all challenges, but also transforms and transforms, adapting to any needs of the baby. It’s great when the period of bearing a child passes easily, without such unpleasant surprises as papillomas during pregnancy.
But if epidermal tumors do appear, the expectant mother faces an acute question regarding the removal of the growth and its effect on the development of the baby. Indeed, is it possible for pregnant women to remove papillomas and why, while expecting a baby, do warts choose the breasts of the expectant mother?
Cystadenopapilloma
Cystadenopapilloma is a papilloma of the mammary gland duct, but when it is located far from the nipple and discharge accumulates in the thickness of the mammary gland, without exiting through the nipple. This papilloma forms a breast cyst with growths.
| Ultrasound signs of peripheral cystadenopapilloma. The papilloma was located in the duct far from the nipple. Discharge (having no outlet) accumulated around her. Read more about such cysts HERE |
Bloody discharge from the nipple
Blood discharge from the nipple of the mammary gland also occurs with cancer. Therefore, it is imperative to perform their microscopic examination.
If the papilloma is inside the cyst, but there is no discharge from the nipple, a puncture (puncture) of the cyst is performed to examine its contents.
The specialists of our Center can take nipple discharge for analysis and perform a biopsy of cystadenopapilloma on the same day of your consultation.
Ductography
Ductography is used to detect papilloma in the mammary duct. To do this, a thin tube (blunt needle) is inserted into the duct (from which discharge is detected), through which a contrast agent is injected into the duct. This drug fills the ducts and “flows around” the papilloma - it becomes noticeable. For small papillomas, the introduction of contrast has a cauterizing effect on them and the discharge may stop. If they appear again, surgery is indicated.
Ductography is a mammography with contrast filling of the breast ducts.